Prostaglandin E1 liposome frozen dry powder injection and production technology thereof
A prostaglandin and injection technology, applied in the field of medicine, can solve the problems such as the inability to exert the curative effect well and the large difference in the effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
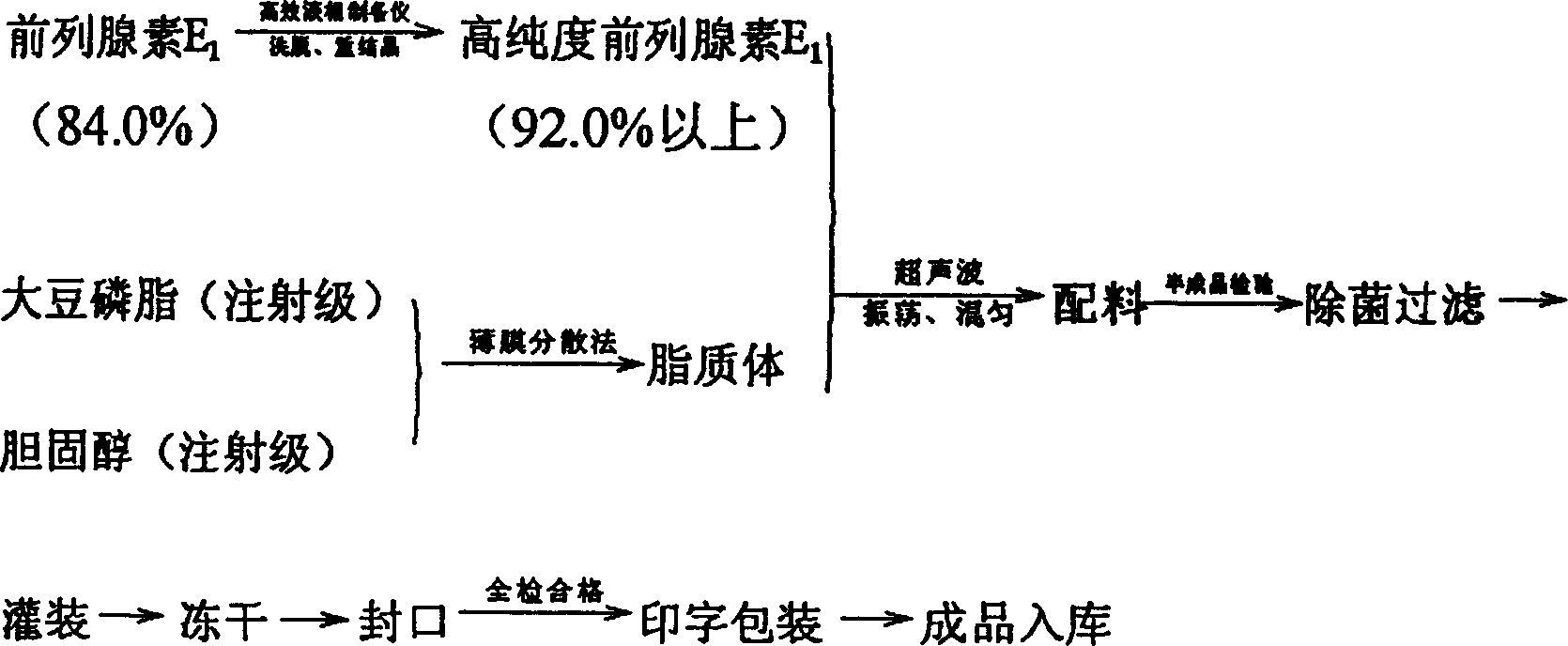
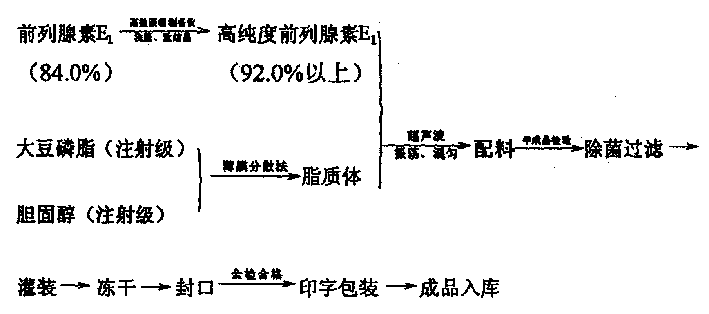
[0032] (1) Prostaglandin E1 with a purity of more than 84.0% is dissolved in chloroform (analytical pure), adsorbed on a high performance liquid phase preparative chromatography column, and through eluent A (chloroform: methanol = 90: 10), Eluent B (chloroform:methanol=95:5) was eluted sequentially, and ethyl acetate (analytical grade) was recrystallized to obtain prostaglandin E1 with a purity of more than 92.0%.
[0033] (2) Dissolve soybean lecithin (injection grade) and cholesterol (injection grade) in chloroform (analytical pure) in a sterile room, and evaporate chloroform under reduced pressure rotation to make lipids form a film on the wall .
[0034] (3) Prostaglandin E1 is prepared in the aseptic room, calculated by 10,000 pieces, weighing 1 gram of high-purity prostaglandin E1 (above 92.0%), dissolving with a small amount of absolute ethanol, and adding phosphate buffer solution of pH 7.8 under stirring (PBS) 1000ml, slowly add the PBS containing prostaglandin E1 in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com