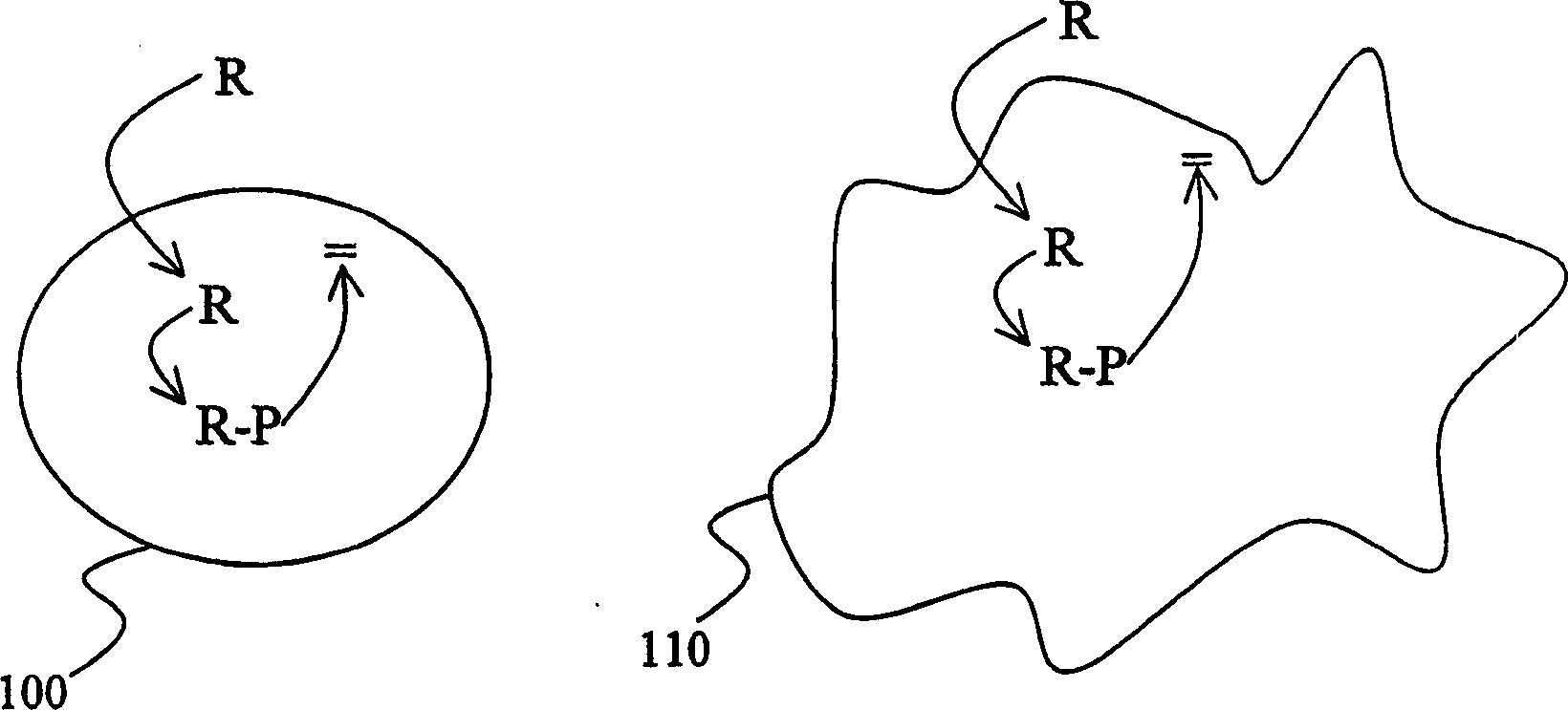
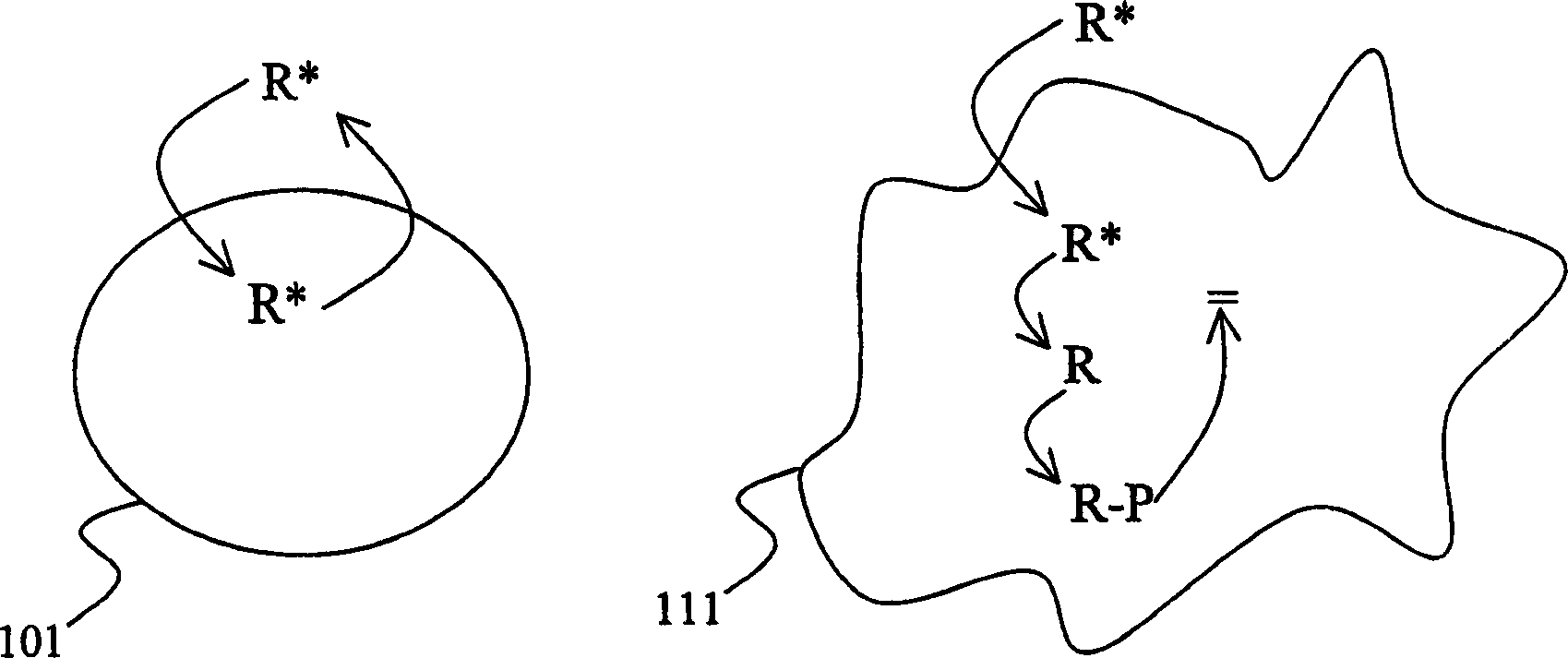
Improved specificity in treatment of diseases
A selective, drug-based technology that can be used to improve specific areas of disease treatment and address issues such as reducing effective doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
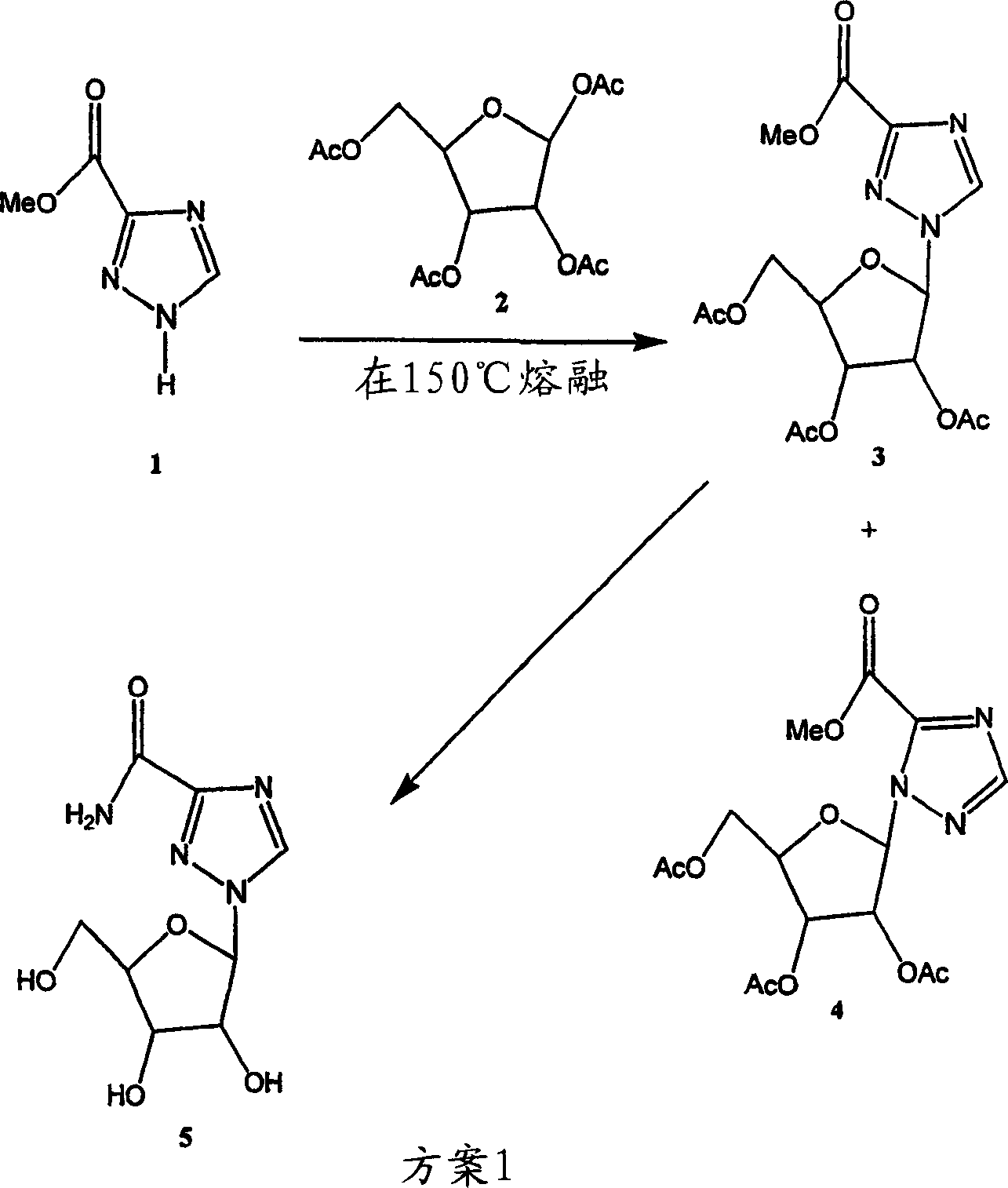
[0052] (a) as attached figure 2 An exemplary synthesis of the indicated ribavirin is shown below.
[0053] Methyl 1-(2,3,5-tri-O-acetyl-β-D-ribofuranosyl)-1,2,4-triazole- 3-Carboxylate (3) and
[0054] Methyl 1-(2,3,5-tri-O-acetyl-β-D-ribofuranosyl)-1,2,4-triazole-5- Carboxylate (4)
[0055] Methyl-1,2,4-triazole-3-carboxylate (25.4 g, 200 mmol) (1), 1,2,3,5-tetra-O-acetyl-β-D-ribofuranose ( A mixture of 63.66 g, 200 mmol) of (2) and bis(p-nitrophenyl)phosphate (1 g) was placed in a RB flask (500 mL). Place the flask in a preheated oil bath at 165-175°C and stir under water vacuum for 25 minutes. Displaced acetic acid was collected in an ice-cold trap placed between the aspirator and the RB flask. Remove the flask from the oil bath and cool. When the flask temperature was about 60-70 °C, EtOAc (300 mL) and saturated NaHCO 3 (150 mL), and extracted with EtOAc. The aqueous layer was extracted with EtOAc (200 mL). The combined EtOAc extracts were washed with satu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com