Use of 2,3-epoxy-1-hydroxy-4,9-radigeranidiolene-12,8:14-diinner-ester as herbicide
A technology of geranadiene and dilactone, applied in 2, can solve the problems of soil and groundwater resource pollution, high residue, slow decomposition of chemical herbicides, etc., and achieve the effect of strong broad-spectrum inhibition.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment one: to the influence of lettuce and ryegrass seed germination rate
[0019] Method: 2,3-epoxy-1-hydroxyl-4,9-geranyadiene-12,8:14,6-dilactone was formulated into 10mg / L, 50mg / L, 100mg / L, 200mg / L, 500mg / L, 1000mg / L several different concentrations, use the same method to test the lettuce and ryegrass seeds, and count the germination rate after 3 days.
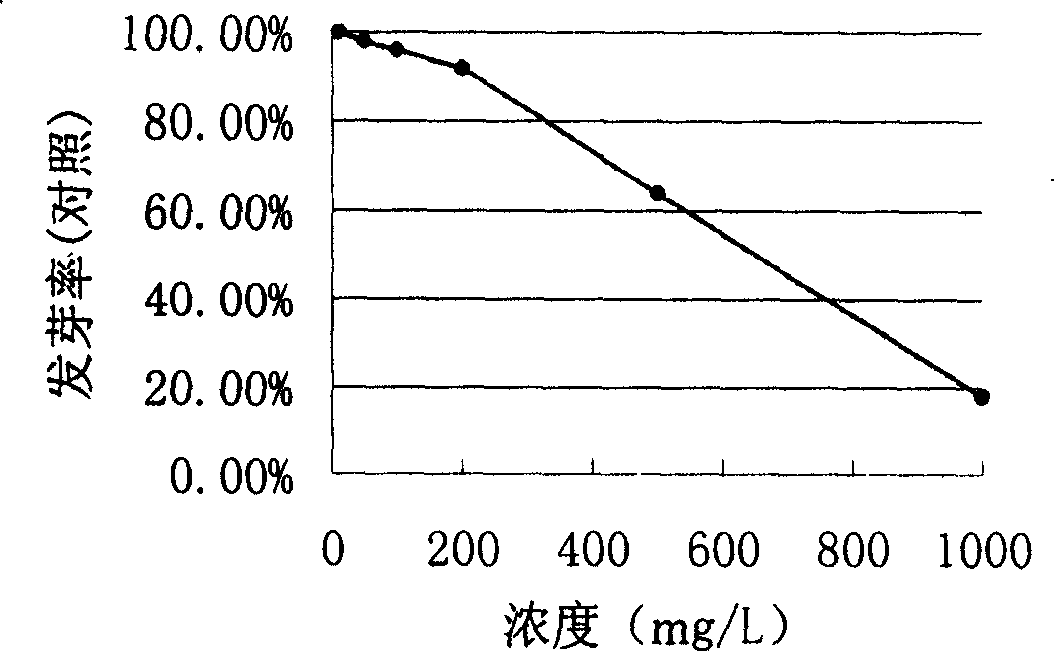
[0020] Results: With the increase of the concentration, the inhibitory effect of 2,3-epoxy-1-hydroxy-4,9-gernadiene-12,8:14,6-dilactone on the germination rate of lettuce seeds gradually increased Big. When the concentration was 10-200mg / L, the germination rate of lettuce seeds was not significantly affected. But when the concentration reached 500mg / L, the germination rate decreased to 64%. When the concentration was 1000mg / L, the lettuce seed germination rate was reduced to 18% (see attached figure 1 ).
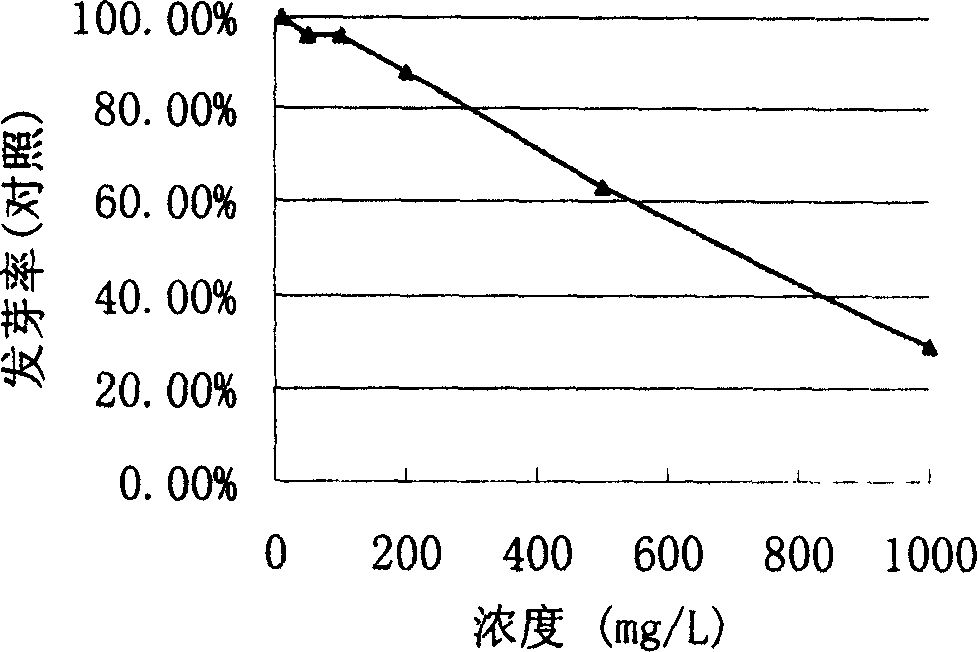
[0021] The effect of 2,3-epoxy-1-hydroxy-4,9-gernadiene-12,8:14,6-dilactone on germination rate of ...
Embodiment 2
[0022] Embodiment two: the impact on the growth of lettuce seedlings and the contrast with commercial herbicide Harness (Harness) effect
[0023] Method: 2,3-epoxy-1-hydroxy-4,9-germanadiene-12,8:14,6-dilactone was formulated into 25mg / L, 50mg / L, 100mg / L, 250mg / L, 500mg / L concentration gradient, the lettuce seeds that had been germinated in advance were used for bioassay, and the root length and seedling height were counted after 3 days. In order to detect its action strength, the herbicide Harness (Herness, produced by Monsanto, USA) was prepared into the same gradient concentration for comparison.
[0024] In order to eliminate the influence brought by the too high or too low pH value in the experiment, the pH value of the solution in the petri dish was measured, compared with the control (distilled water), the pH value was basically the same, all around 6.0.
[0025] Results: 2,3-Epoxy-1-hydroxyl-4,9-gernadiene-12,8:14,6-dilactone had an IC value of half inhibitory concen...
Embodiment 3
[0028] Example 3: Effect of 2,3-epoxy-1-hydroxyl-4,9-geranyadiene-12,8:14,6-dilactone on the growth of ryegrass seedlings
[0029] Method: same as embodiment two
[0030] Results: Same as the effect on lettuce seedlings, the growth of ryegrass was significantly inhibited by 2,3-epoxy-1-hydroxy-4,9-gernadiene-12,8:14,6-dilactone. The half inhibitory concentration IC of 2,3-epoxy-1-hydroxy-4,9-geranyadiene-12,8:14,6-dilactone on the root growth of ryegrass seedlings 60 The value is 290mg / L (see attached Figure 5 ). At higher concentrations, 2,3-epoxy-1-hydroxy-4,9-gernadiene-12,8:14,6-dilactone yellowed the root tips of seedlings. Likewise, 2,3-epoxy-1-hydroxy-4,9-gernadiene-12,8:14,6-dilactone had a much weaker effect on shoot height than root length in ryegrass (see attached Figure 6 ).
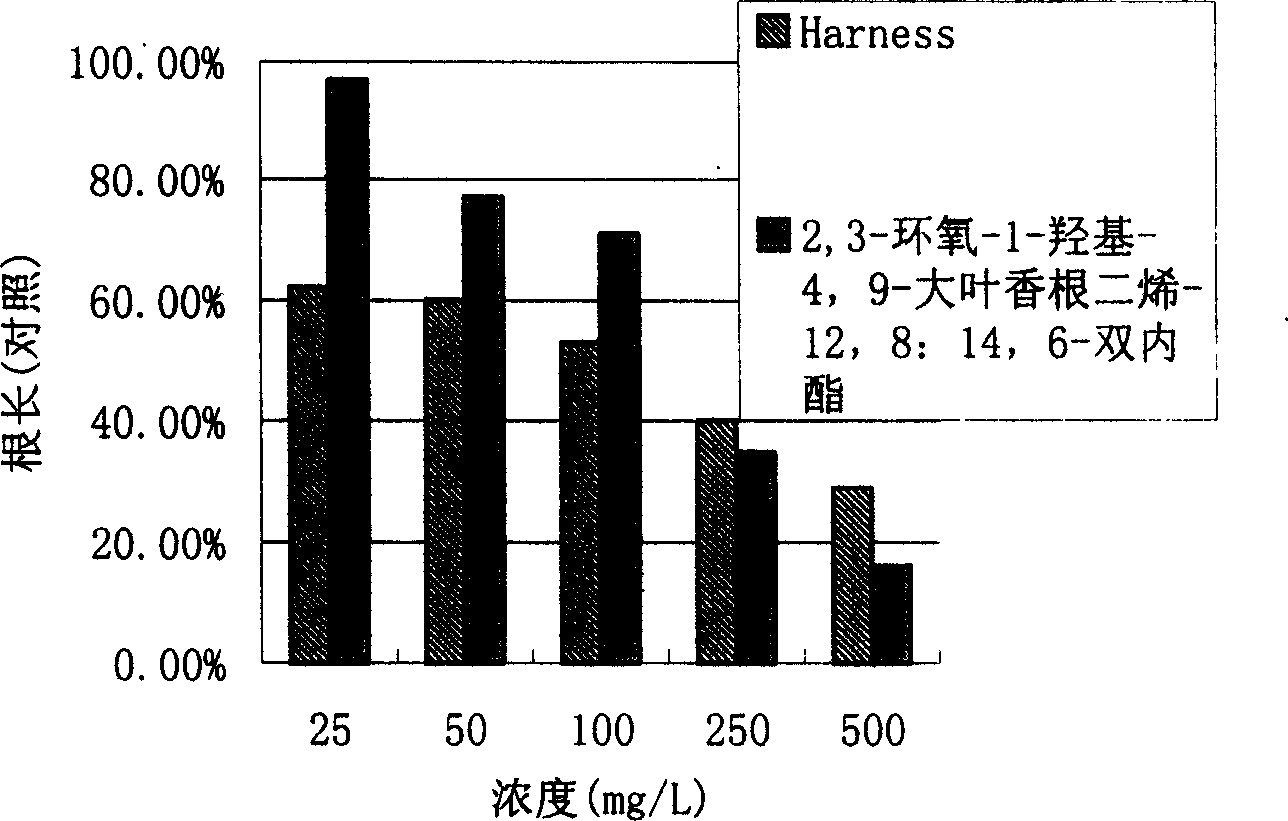
[0031]The comparison with the commercial chemical herbicide Harness (Harness) shows that when the concentration is greater than or equal to 50mg / L, 2,3-epoxy-1-hydroxy-4,9-bigergeradi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com