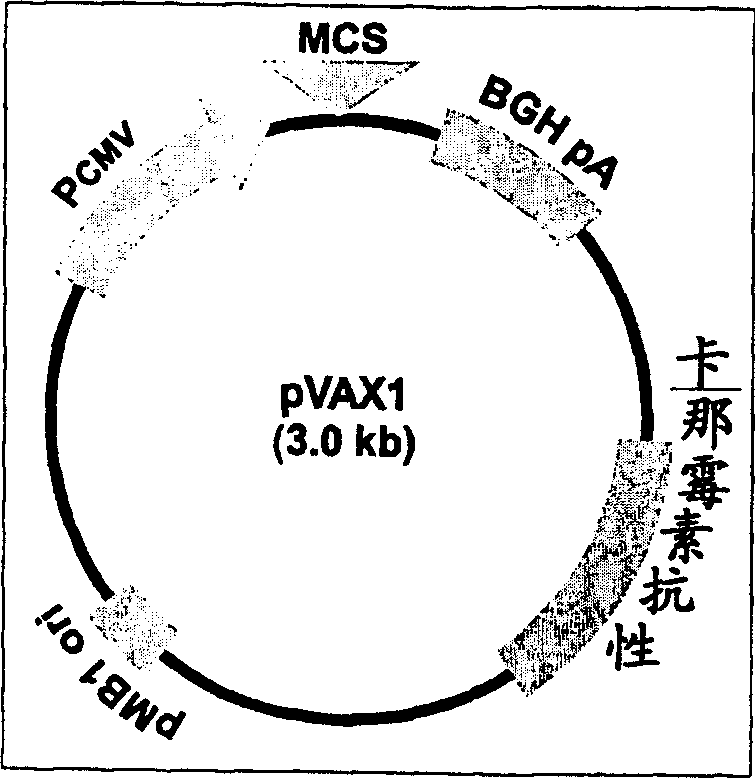
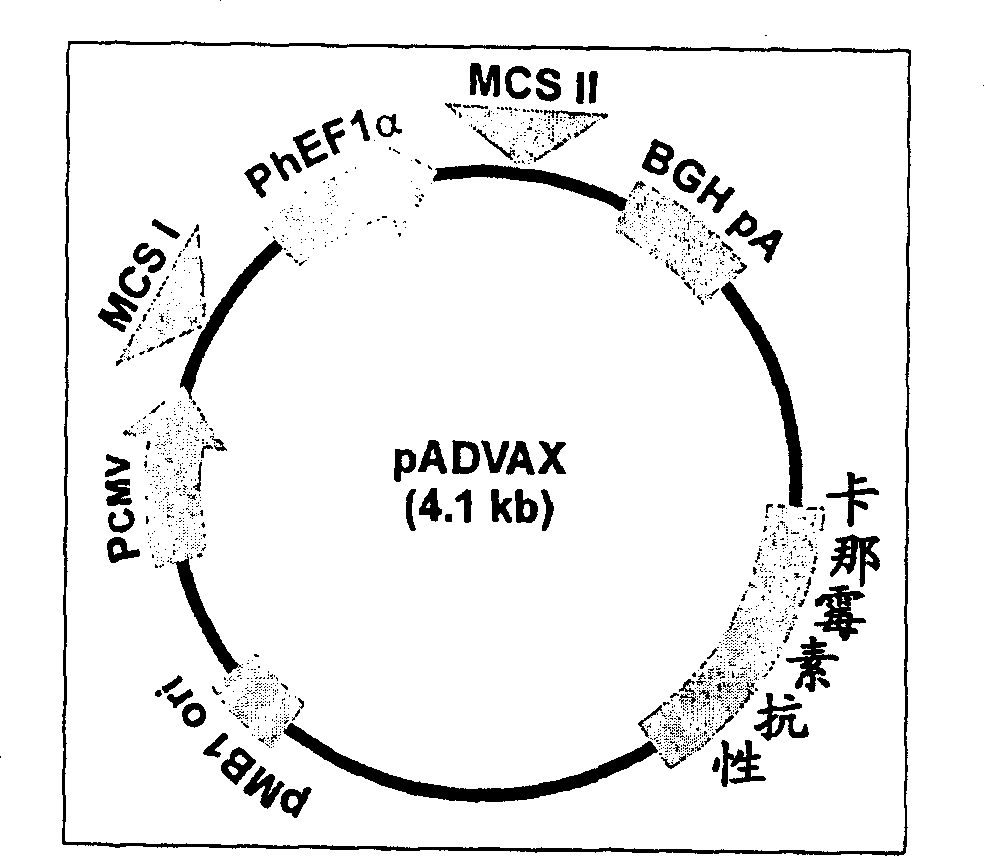
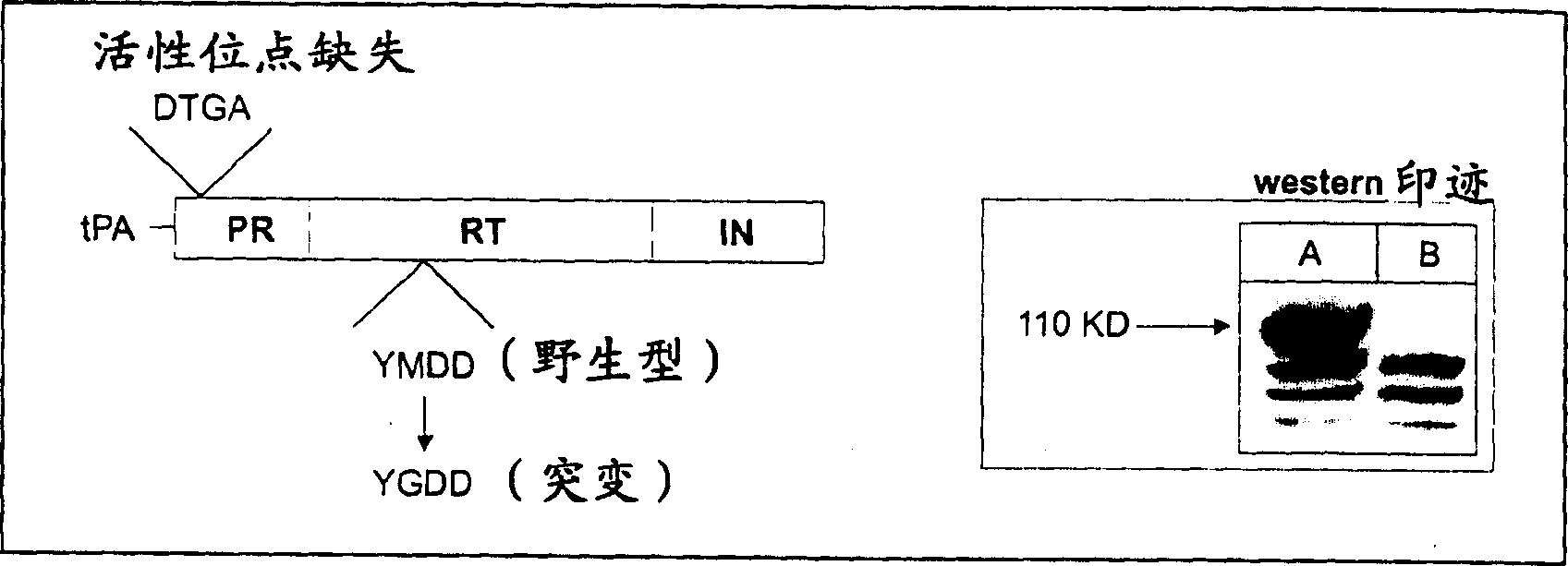
Immune method and composition for HIV-1
A technology of HIV-1 and composition, applied in the field of DNA vaccines, which can solve the problems of unacceptable, dangerous, ineffective infection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0068] The following examples are provided to provide those of ordinary skill in the art with a complete disclosure and description of how to make and use the assay, screening and treatment methods of the invention and are not intended to limit the scope of what the inventors believe to be their invention. Efforts have been made to ensure accuracy with respect to data used (eg amounts, temperature, etc.), however, some experimental error and error should be accounted for. Unless indicated otherwise, parts are parts by weight, molecular weight is average molecular weight, temperature is in degrees Centigrade, and pressure is at or near atmospheric.
[0069] Example 1. Protocol: Direct ELISA Measurement of Anti-HIV-1 gag Antibodies in Immunized Animals
[0070] Gag protein-sample-anti-mouse IgG.ALP . Dissolve in 0.1M NaHCO with 100 μl 3 Gag protein (0.5 μg / well) in solution (pH 9.6) was coated with culture plates overnight at 4°C (Immulon-2, Dynex Technologies, Chantilly, VA...
Embodiment 2
[0071] Example 2. Competitive gp120-sCD4 ELISA protocol
[0072] Anti-gp120Abs-gp120-sCD4.IgG-anti-human IgG.ALP . Dissolved in 0.1M NaHCO 3 Anti-gp120 antibody in solution (pH 8.6) was used to coat the culture plate overnight at room temperature. The plate was washed twice with PBS, and then blocked by adding PBS containing 4% skimmed milk powder and 0.5% BSA for 1 hour at room temperature. Mix the blocking solution, add the gp120 supernatant, and react at room temperature for 1 hour. Plates were washed 4 times with PBS containing Tween-20. Add sCD4 or control sterilely diluted in blocking buffer and incubate the plate for 1 hour at room temperature. Plates were washed 4 times with PBS containing Tween-20. A fixed concentration of sCD4-human IgG was added and the plate was incubated for 1 hour at room temperature. Plates were washed 4 times with PBS containing Tween-20. Alkaline phosphatase-labeled anti-human IgG was added and the plate was incubated for 30 minutes a...
Embodiment 3
[0073] Example 3. Mouse IFN-γ ELISpot test
[0074] Day 1 . ELISpot filter plates were pre-coated by adding capture antibody (eg mouse IFN-γ) diluted 1:50 in coating buffer (eg 125 μl antibody in 5 ml coating buffer). 100 μl of capture antibody / coating buffer was placed in each well, covered and incubated overnight at 4°C.
[0075] day 2 . Cells were harvested and 4 plates were seeded with PBST. Each well was blocked with R10 (200 μl / well) at 37° C. for 2 hours. Add cells according to the plan for the specific plate. Add peptide, and in CO 2 Incubate overnight at 37°C in an incubator.
[0076] 3rd day . Plates were washed 5 times with PBST, then 100 [mu]l per well of detection antibody diluted 1:60 in 1% BSA was added. Plates were incubated overnight at 4°C.
[0077] day 4 . The plate was washed 4 times with PBST, and then 100 [mu]l of SAP diluted 1:60 in 1% BSA was added to each well. Plates were incubated at room temperature for 2 hours, washed 4 times wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com