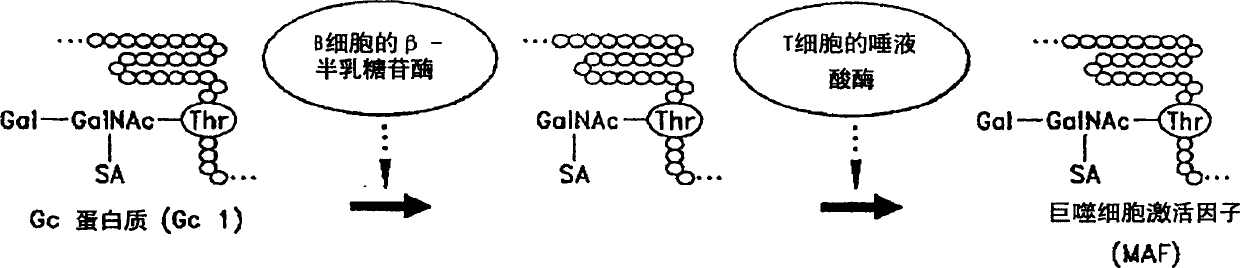
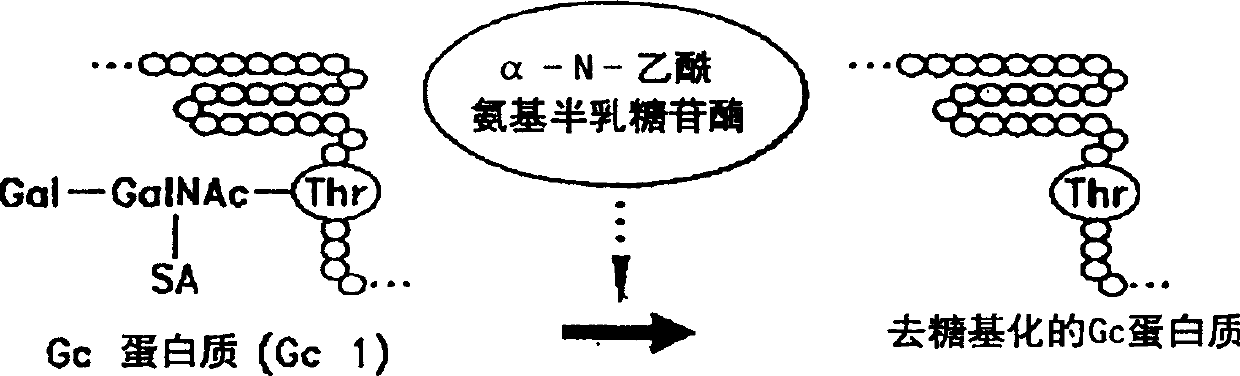
Determination of alpha-N-acetamino galactosidase activity
A technology of acetylaminosemi-glycosidase, applied in the direction of organic active ingredients, hydrolase, specific peptides, etc., can solve the problem of decreased activity of plasma Gc protein precursor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
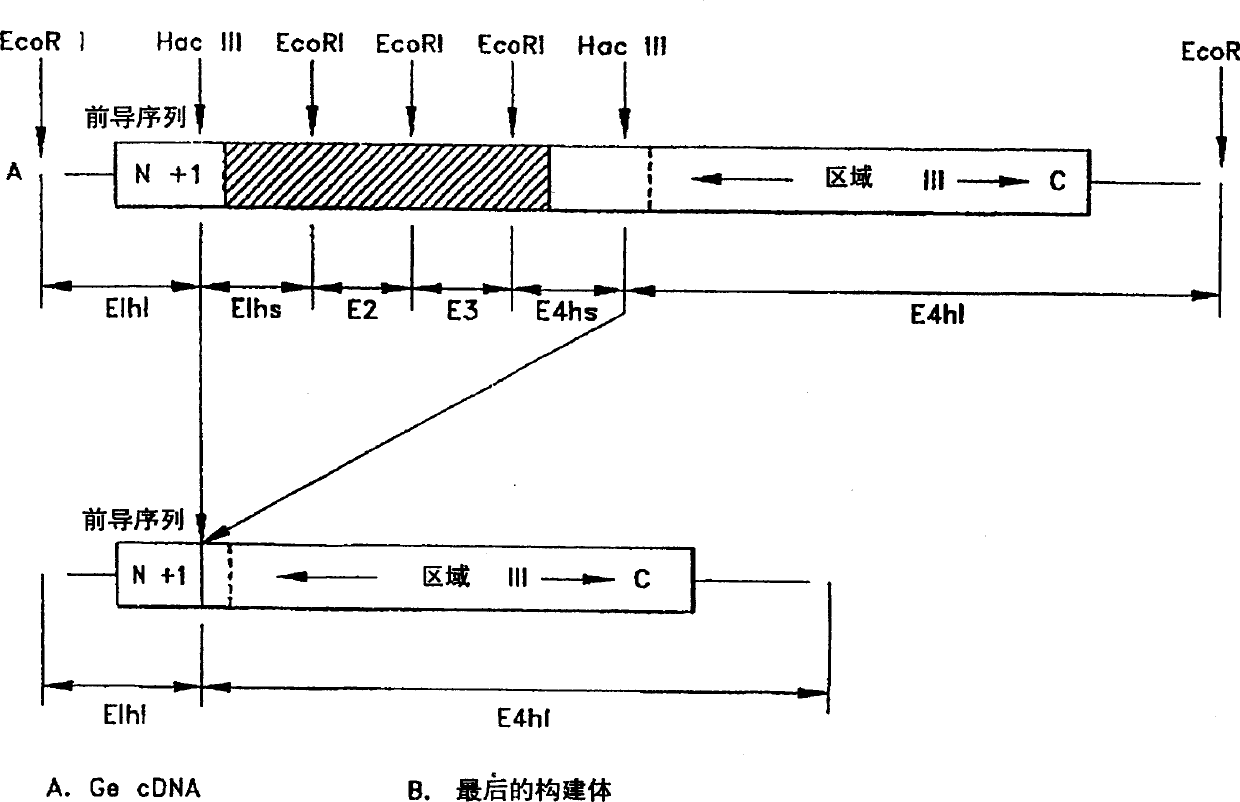
[0040] Description of method for cloning of gene encoding macrophage activating factor
[0041] A. Cloning the cDNA of the Gc protein into an insect virus
[0042] The full-length cDNA of bm human Gc protein was isolated from the human liver cDNA library of bacteriophage lambda gtll (Clontech, Palo Alto, CA) using the pico BlueTm immunoscreening kit from Stratagene of La Jolla, CA. There are several facts that illustrate the advantages of the polyhedrin expression system of the baculovirus in insect cells: (a) in the later stages of the infection cycle, the system contains more than half of the total cellular protein and is expressed at very high levels in infected cells (b) is not necessary for viral infection or replication, meaning that the recombinant virus does not require any auxiliary functions; (c) the virus lacking the polyhedron gene has a special plaque morphology derived from the virus containing the cloned gene; (d) Unlike bacterial cells, insect cells efficientl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com