Nicotine-containing oral dosage form
An oral dose and form technology, applied in biocides, pill delivery, animal repellants, etc., can solve the problem of rapid release of addiction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Nicotine lozenges were prepared as follows. In a 500ml beaker, combine 100g of ISOMALT powder, 25g of water and 0.4g of menthol. Heat the mixture using a hot plate while stirring until all of the ISOMALT is melted. Continue mixing and heating to about 165°C. Continue stirring and lower the temperature to about 120°C. At about 120°C, add about 1.2 g of sodium carbonate to adjust the pH to about 7.5-9.0 (pH can be measured from a solution of 0.1 g of this mixture in 10 ml of deionized water), and all other desired optional ingredients such as flavoring and / or vitamins. At about 120°C, 185 g of nicotine bitartrate dihydrate (equivalent to 60 mg of nicotine free base) was added to the mixture, mixed well and the molten mixture maintained at about 120°C. This mixture is pushed through a confectionery former to produce nicotine lozenges. Alternatively, the mixture may be deposited in suitable molds, cooled and demolded to obtain nicotine lozenges. By the time it solidif...
Embodiment 2
[0078] Mix 75% ISOMALT and 25% purified water by weight. While mixing, heat the ISOMALT / water mixture until all the ISOMALT is melted. Continue mixing and heating to about 165°C. Continue mixing and lower the temperature to about 120°C. At about 120°C, sodium carbonate was added to adjust the pH to about 7.5-9.0 (pH can be measured on a solution of 0.1 g of this mixture dissolved in 10 ml of deionized water), nicotine component and sesame oil. The molten mixture is maintained at about 120°C and formed into lozenges using suitable molds.
[0079] By the time the tablet has set, most of the processing water will have evaporated, leaving only residual water.
Embodiment 3
[0081] The in vitro dissolution profile of the lozenges was determined using a VanKel model VK7000 Dissolution Bath under the following conditions:
[0082] a.USP instrument I (Basket)
[0083] b. Dissolving medium: 900ml USP phosphate buffer (pH=7.4)
[0084] c. Dissolving temperature: 37℃+ / -0.5℃
[0085] d. Shaft peripheral speed: 100rpm
[0086] e. per container, each predetermined time interval (e.g. 5, 10, 20 and 30 minutes,
[0087] and 1, 2, 3, 4, 5, 6, 7 and 8 hours until 100% or steady state release
[0088] put), collect a 2ml sample with an automatic sampling device. Use 2ml for each time interval
[0089] Phosphate buffered saline replaces the removed media.
[0090] f. directly analyzing the nicotine content of the sample by HPLC method.
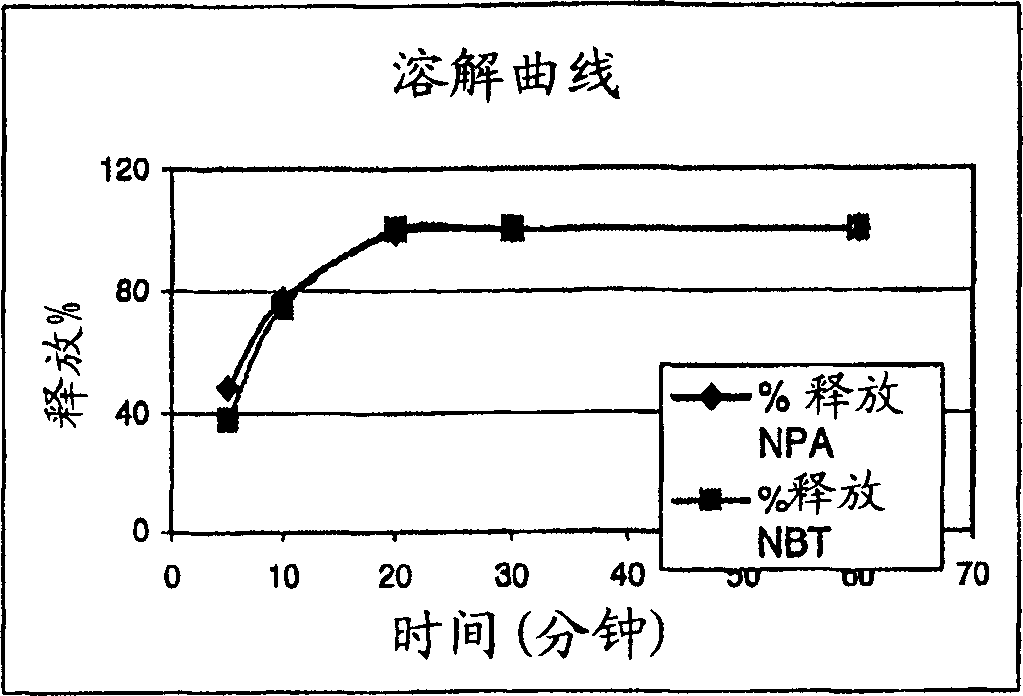
[0091] The in vitro dissolution curve of the lozenge prepared according to Example 2 is shown in figure 1 . Such as figure 1 As shown, the nicotine is completely released within about 20 minutes; at least about 50% is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com