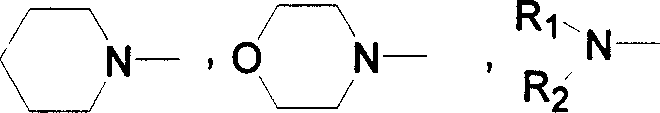
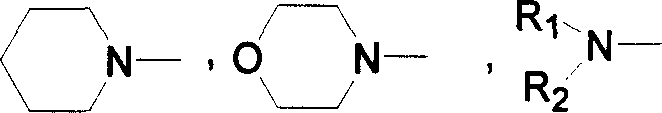
Derivant of electrophosphorescence 1, 8-naphthimide and luminous ligand thereof
A kind of naphthalimide, electrophosphorescence technology, applied in 1 field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
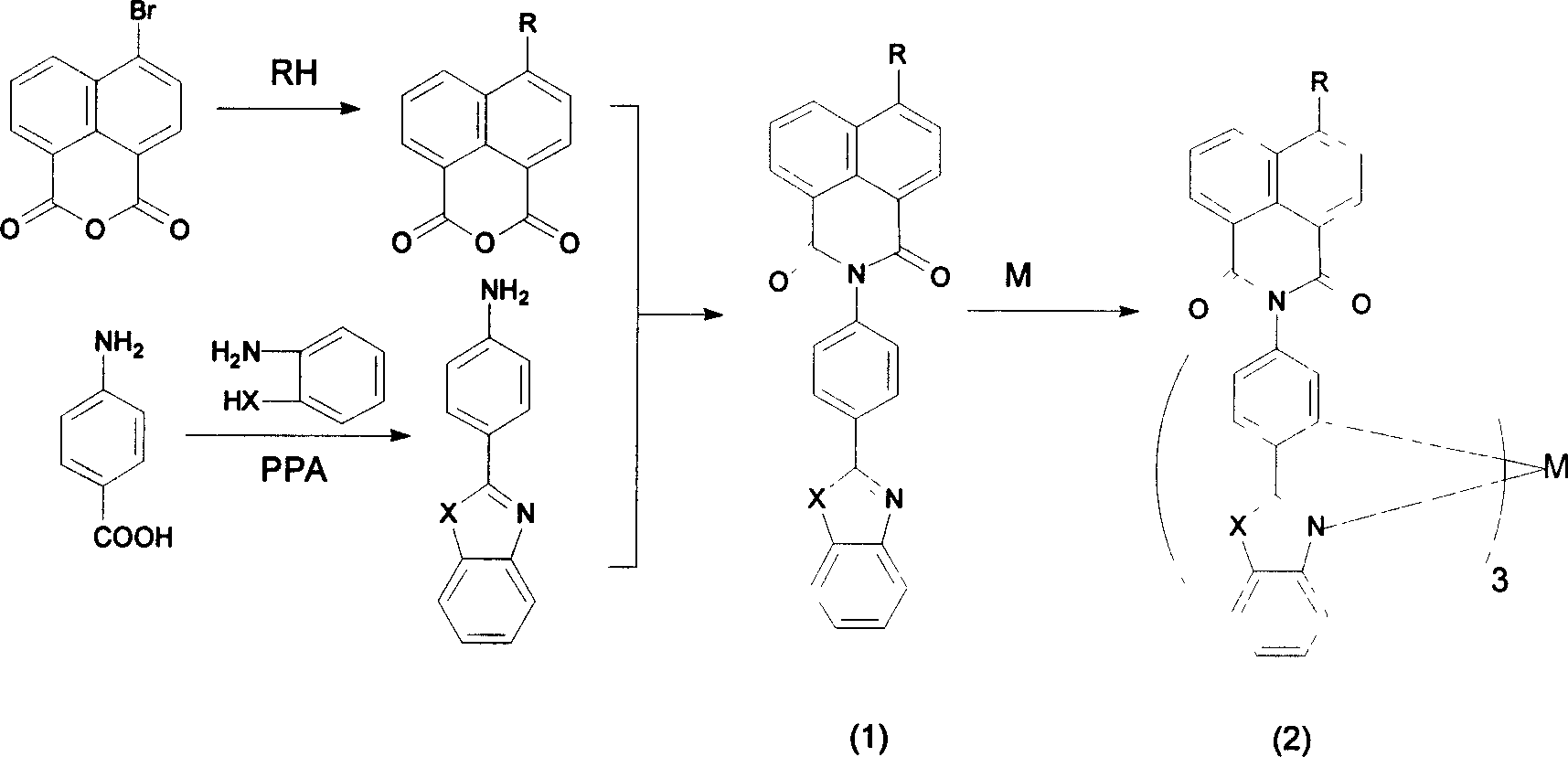
[0025] Synthesis of 4-piperidinyl-N-[4-(2-benzothiazolyl)phenyl]-1,8-naphthalimide:
[0026]
[0027] (1) Preparation of 4-piperidinyl-1,8-naphthalene anhydride
[0028] In a round bottom flask, add 2.76g of 4-bromo-1,8-naphthalene anhydride, 1.5mL of piperidine and 40mL of ethylene glycol monomethyl ether, stir, and heat to reflux for 3h. After standing overnight, a precipitate precipitated out. Suction filtration yielded an orange solid. Recrystallized from ethanol and ethylene glycol monomethyl ether to obtain 2.04 g of orange-red needle-like crystals with a yield of 73.6%. The melting point is 172-173°C.
[0029] (2) Preparation of 4-(2-benzothiazolyl)aniline
[0030] In a round bottom flask, 2.74 g p-aminobenzoic acid, 2.2 mL o-aminothiophenol and 30 mL polyphosphoric acid (PPA) were added. Protected by argon, heated to 250°C for 4h. After cooling, dilute with water until solid precipitates. Filter, pour the filter cake into 10% Na 2 CO 3 In the aqueous soluti...
Embodiment 2
[0037] Synthesis of 4-piperidinyl-N-[4-(2-oxazolyl)phenyl]-1,8-naphthalimide:
[0038]
[0039] (1) Preparation of 4-(2-benzoxazolyl)aniline
[0040] Except that one of the raw materials o-aminothiophenol is replaced by o-aminophenol, the preparation method is the same as (2) in Example 1. 3.99 g of pure product was obtained with a yield of 63%. The melting point is 172-175°C.
[0041] (2) Preparation of 4-piperidinyl-N-[4-(2-oxazolyl)phenyl]-1,8-naphthalimide
[0042] 4-(2-benzoxazolyl)aniline and 4-piperidinyl-1,8-naphthalene anhydride (see step (1) of Example 1 for preparation) were prepared according to the method of (3) in Example 1 Product (yellow-green solid). Yield 45.8%. Melting point > 270°C.
[0043] 1 H-NMR (CDCl 3 ): 1.76(m, 2H), 1.92(m, 4H), 3.28(m, 4H), 7.22(d, J=8.08Hz, 1H), 7.39(t, 2H), 7.50(d, J=8.43Hz , 2H), 7.62(d×d, J=3.36Hz, J=2.29Hz, 1H), 7.73(t, 1H), 7.82(d×d, J=2.33Hz, J=3.3Hz, 1H), 8.44 (d, J=8.43Hz, 2H), 8.46(t, 1H), 8.56(d, J=8.06Hz, 1H...
Embodiment 3
[0046] Synthesis of 4-piperidinyl-N-[4-(2-benzimidazolyl)phenyl]-1,8-naphthalimide:
[0047]
[0048] (1) Preparation of 4-(2-benzimidazolyl)aniline
[0049] Except that one of the raw materials o-aminothiophenol is replaced by o-phenylenediamine, the preparation method is the same as (2) in Example 1. 1.80 g of pure 4-(2-benzimidazolyl)aniline was obtained with a yield of 28.7%. The melting point is 174-175°C.
[0050] (2) Preparation of 4-piperidinyl-N-[4-(2-benzimidazolyl)phenyl]-1,8-naphthalimide
[0051] 4-(2-benzimidazolyl)aniline and 4-piperidinyl-1,8-naphthalene anhydride (see step (1) of Example 1 for preparation) were prepared according to the method of (3) in Example 1 to obtain the target product (yellow-green solid). Yield 55.1%. Melting point > 270°C.
[0052] 1 H-NMR (CDCl 3 ): 1.76(m, 2H), 1.92(d, J=4.59Hz, 4H), 3.30(m, 4H), 7.25(t, 1H), 7.27(d, 2H), 7.44(d, J=8.21Hz , 2H), 7.47(t, 1H), 7.74(t, 1H), 7.84(d, J=6.31Hz, 1H), 8.13(d, J=8.2Hz, 2H), 8.48(...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com