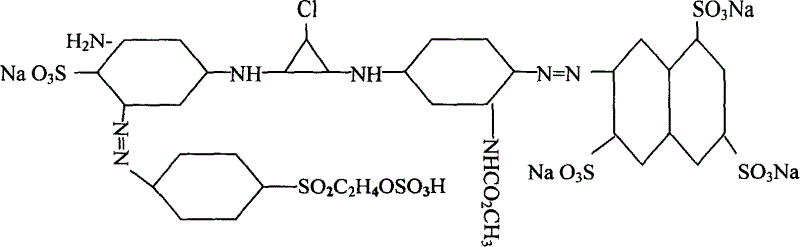
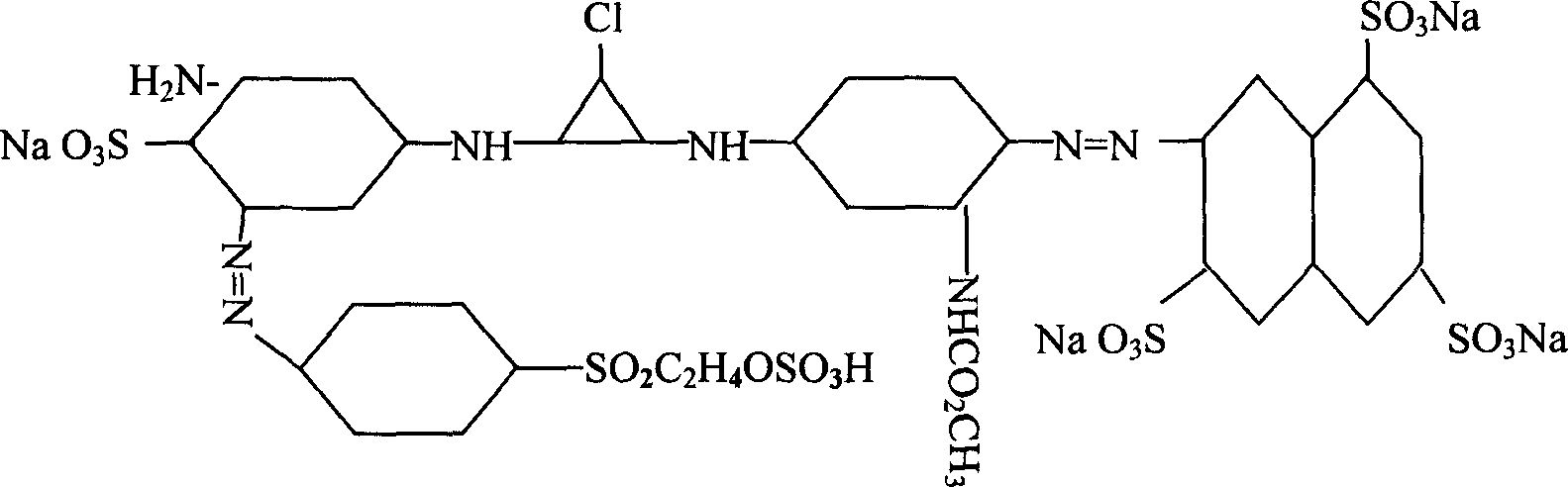
Active yellow SHE, synthetic method and its composite active yellow dye
A synthetic method and reactive yellow technology, applied in the field of dyes, can solve the problems of dyes that cannot dye dark colors, high unit cost, waste of materials, etc., and achieve the effects of reducing production costs, reducing printing and dyeing costs, and eliminating environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Diazo: Beat 100 parts of 3,6,8-trisulfonic acid-2-naphthylamine dry product with ice, then add hydrochloric acid with a ratio of 1-1.5, add sodium nitrite solution to carry out diazo, and control the temperature at 0- 10°C.
[0011] Coupling: Add 100 parts of m-aminoaniline acetylated hydrochloride to the above diazo compound for coupling, pH value 4-7, temperature 5-10°C.
[0012] Primary condensation: ice grind 105 parts of cyanuric chloride at about -2-2°C, then add the above-mentioned coupling material for primary condensation, control temperature at 5-15°C, and pH value at 5-7.
[0013] Para-ester diazo coupling: beat 85 parts of dry p-(β-sulfate ethyl sulfone) aniline in the presence of ice, then add hydrochloric acid with a ratio of 1-1.5, and carry out diazo with sub-nano solution, At the end of the diazo, 2,4-disulfonic acid aniline was added for coupling.
[0014] Secondary condensation: add the primary condensation material to the above-mentioned coupling m...
Embodiment 2
[0015] Example 2, referring to Example 1, during the second condensation, 95 parts of the coupling solution of para-ester diazonium salt and 2,4-disulfonic acid aniline are subjected to secondary condensation, and the pH is controlled at 7-9, and the temperature is 40-60 °C, spray dry after the reaction, and standardize.
Embodiment 3
[0016] Example 3, composite reactive yellow, consists of 90 parts of reactive yellow SHE monomer, 1 part of dustproof agent, and 9 parts of sodium sulfate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com