Quality determination method for external cultivated bezoar
A technology for quality identification and bezoar, applied in the quality field of cultivating bezoar in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
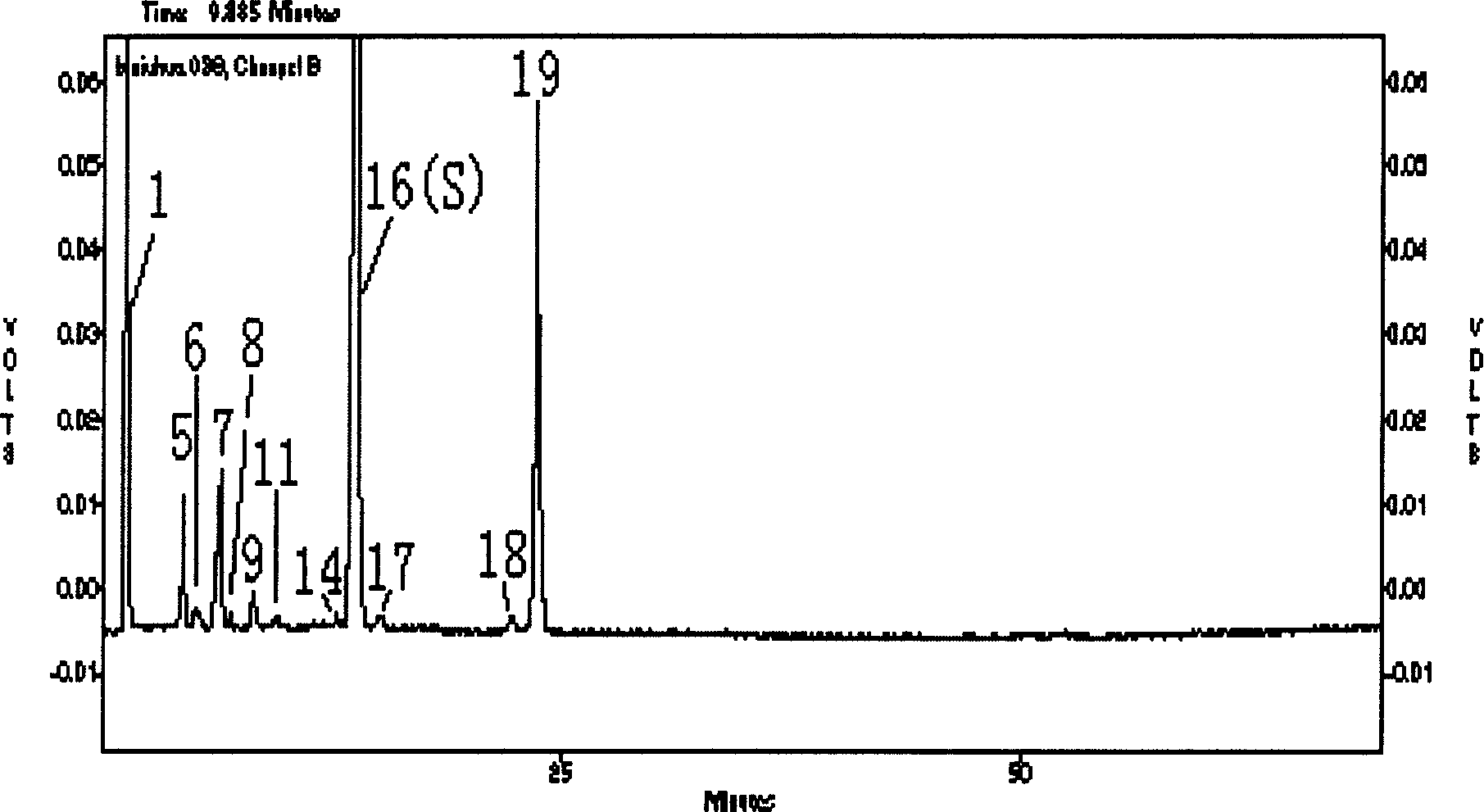
[0048] (1) The research method of standard fingerprint of bile acid components of bezoar cultured in vitro
[0049] 1. Preparation of the test solution
[0050] Take 10 batches of in vitro cultured bezoar fine powder or take 0.2 g of preparation powder containing in vitro cultured bezoar, weigh them accurately respectively, add 10.0 ml of a mixed solution of 0-5% formic acid aqueous solution and methanol (v:v=5:95) , placed in a stoppered Erlenmeyer flask for ultrasonic extraction for 10-45 minutes, the extract was placed in a centrifuge tube, centrifuged, the supernatant was filtered through a 0.45 μm filter membrane, the initial filtrate was discarded, and the subsequent filtrate was obtained.
[0051] 2. Preparation of reference solution
[0052]Take an appropriate amount of reference substances of cholic acid and deoxycholic acid, weigh them accurately, dissolve them in methanol, and prepare solutions containing 1 mg of reference substances per ml.
[0053] 3. Detection ...
Embodiment 2
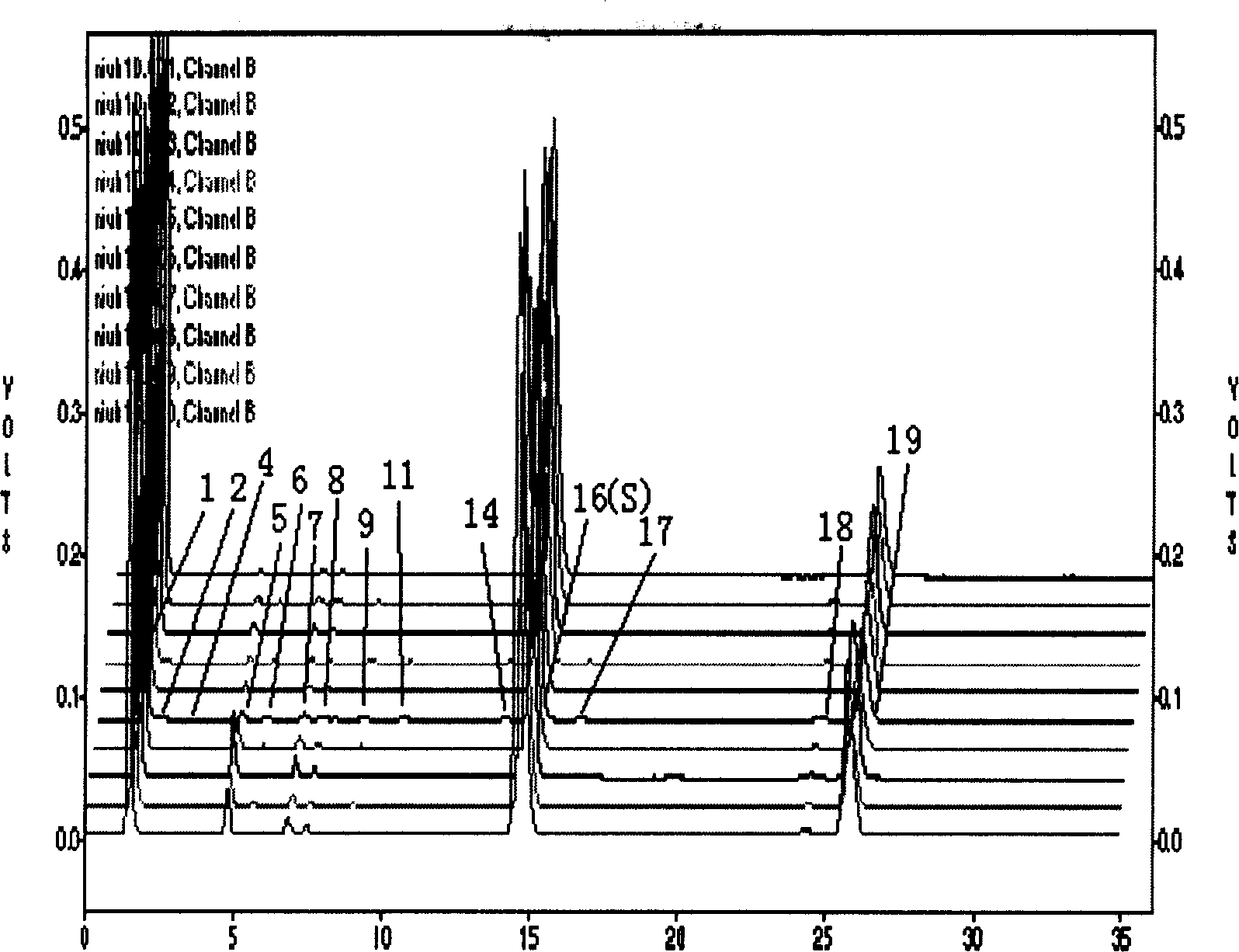
[0098] Embodiment 2, a kind of quality identification method of in vitro cultured bezoar,
[0099] (1) Establishment of fingerprints of bile acids in vitro cultured bezoar
[0100] a, preparation of test solution
[0101] Take more than 10 batches of fine powder of in vitro cultured bezoar or preparation powder containing in vitro cultured bezoar, and accurately weigh them respectively. Add 100 times the amount (ml / g) of a mixed solution of 5% organic acid acetic acid aqueous solution and low-carbon organic alcohol ethanol, put it in a stoppered Erlenmeyer flask for ultrasonic extraction for 10-45 minutes, put the extract in a centrifuge tube, centrifuge, and The clear liquid is filtered with a 0.45 μm filter membrane, the initial filtrate is discarded, and the subsequent filtrate is taken to obtain,
[0102] Wherein the volume ratio of organic acid aqueous solution and low carbon organic alcohol is 5: 95,
[0103] b. Preparation of reference substance solution
[0104] Ta...
Embodiment 3
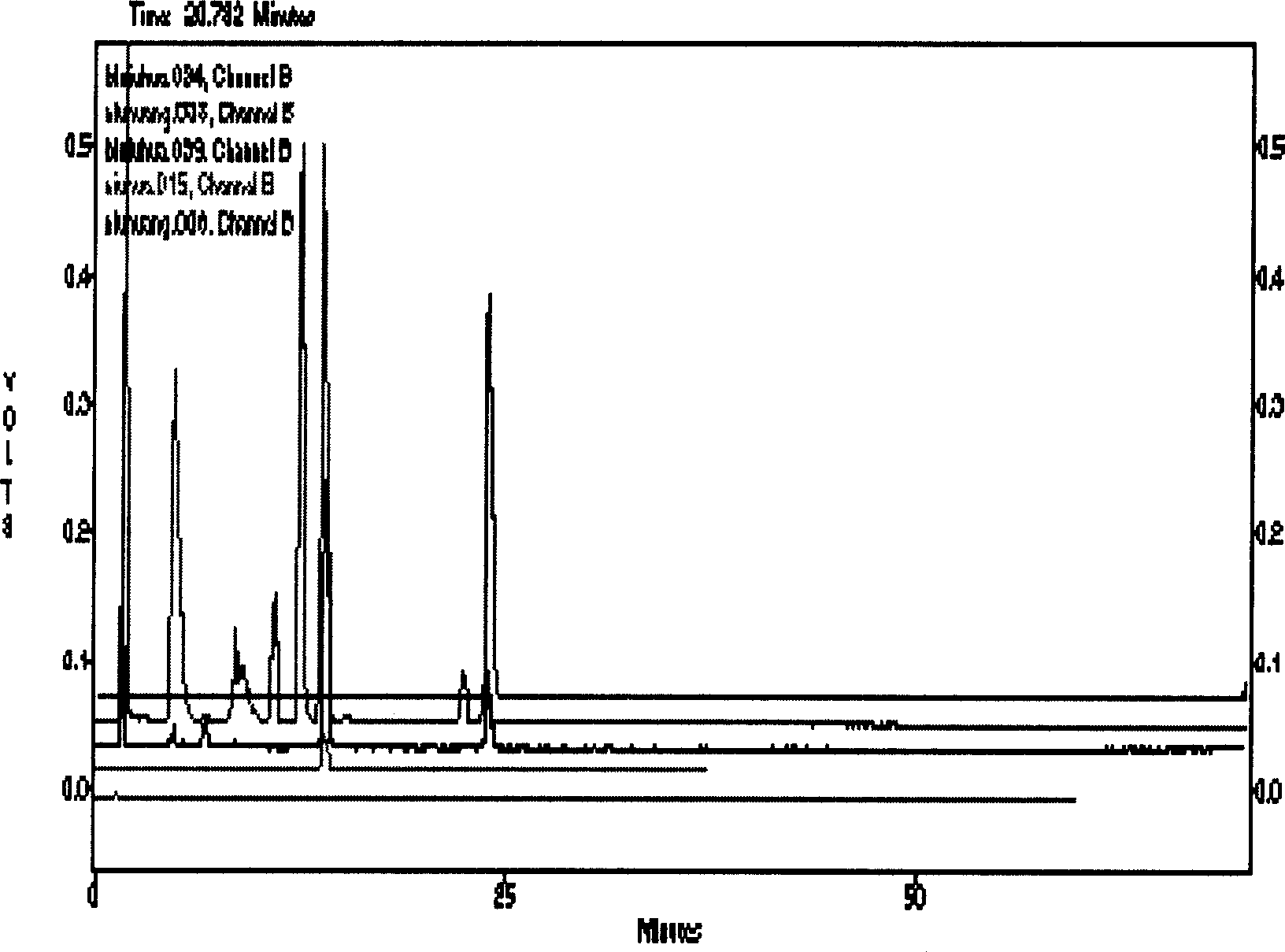
[0124] A method for identifying the quality of in vitro cultured bezoar,
[0125] (1) Establishment of fingerprints of bile acids in vitro cultured bezoar
[0126] a, preparation of test solution
[0127] Take more than 10 batches of fine powder of in vitro cultured bezoar or preparation powder containing in vitro cultured bezoar, and accurately weigh them respectively. Add 20 times the amount (ml / g) of the mixed solution of 2% organic acid trifluoroacetic acid aqueous solution and low-carbon organic alcohol n-butanol 10.0ml, put it in a stoppered Erlenmeyer flask for ultrasonic extraction for 10-45min, and put the extract in a centrifuge tube , centrifuged, and the supernatant was filtered with a 0.45 μm filter membrane, the initial filtrate was discarded, and the subsequent filtrate was taken to obtain,
[0128] Wherein the volume ratio of organic acid aqueous solution and low-carbon organic alcohol is 10:97,
[0129] b. Preparation of reference substance solution
[013...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com