Prostaglandin composition for the treatment of erectile dysfunction
A technology for erectile dysfunction and composition, which is applied in the field of compositions for treating erectile dysfunction, and can solve problems such as the degree of swelling that cannot be reached
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
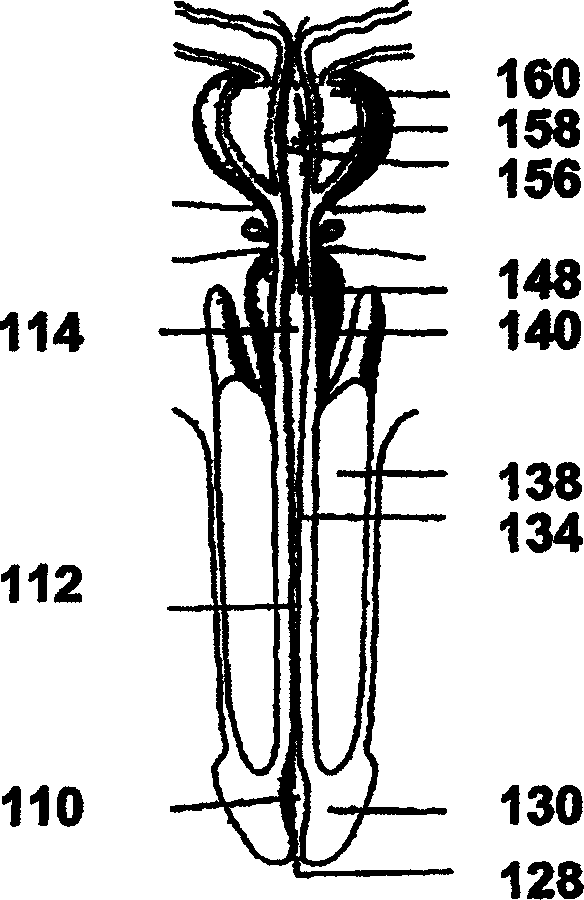
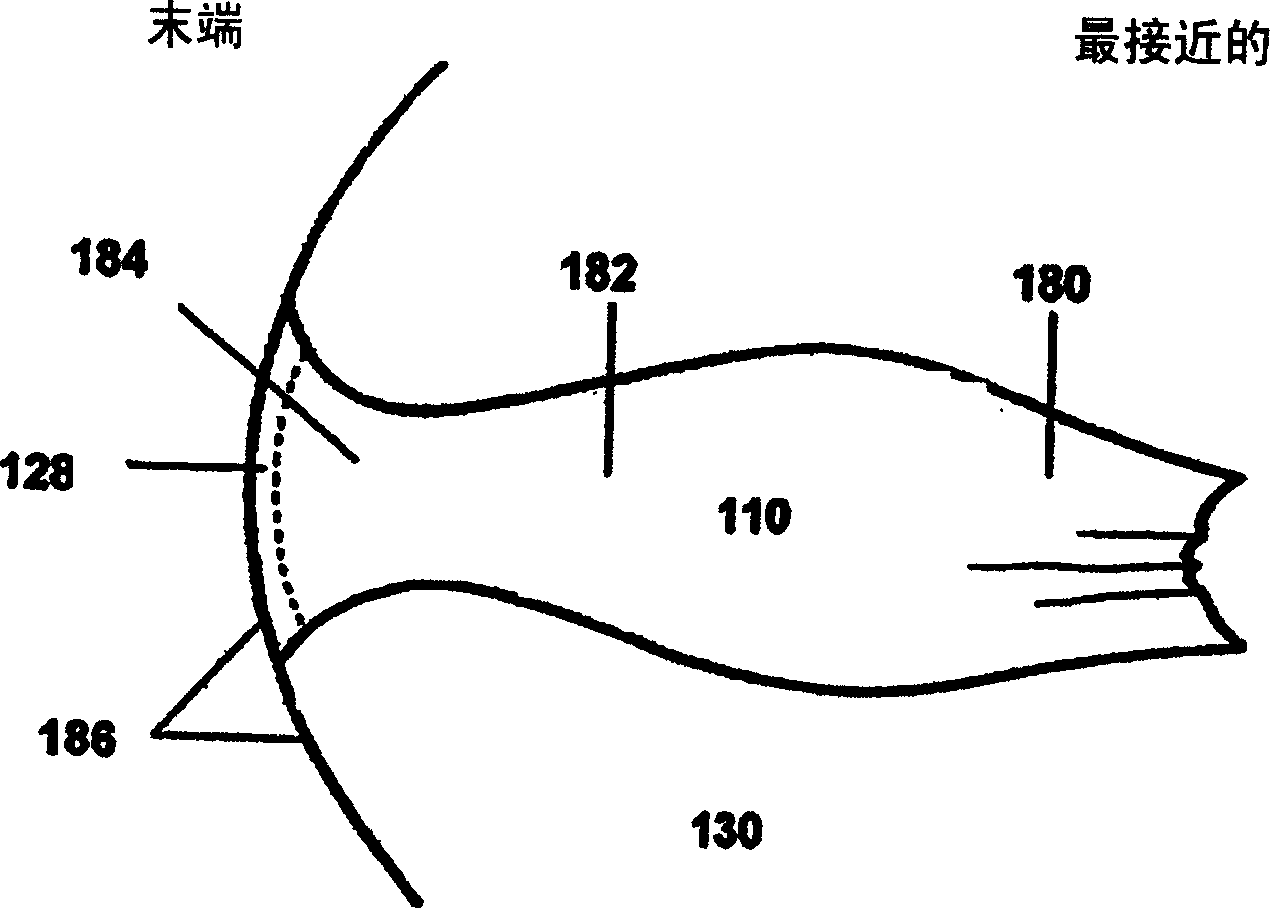
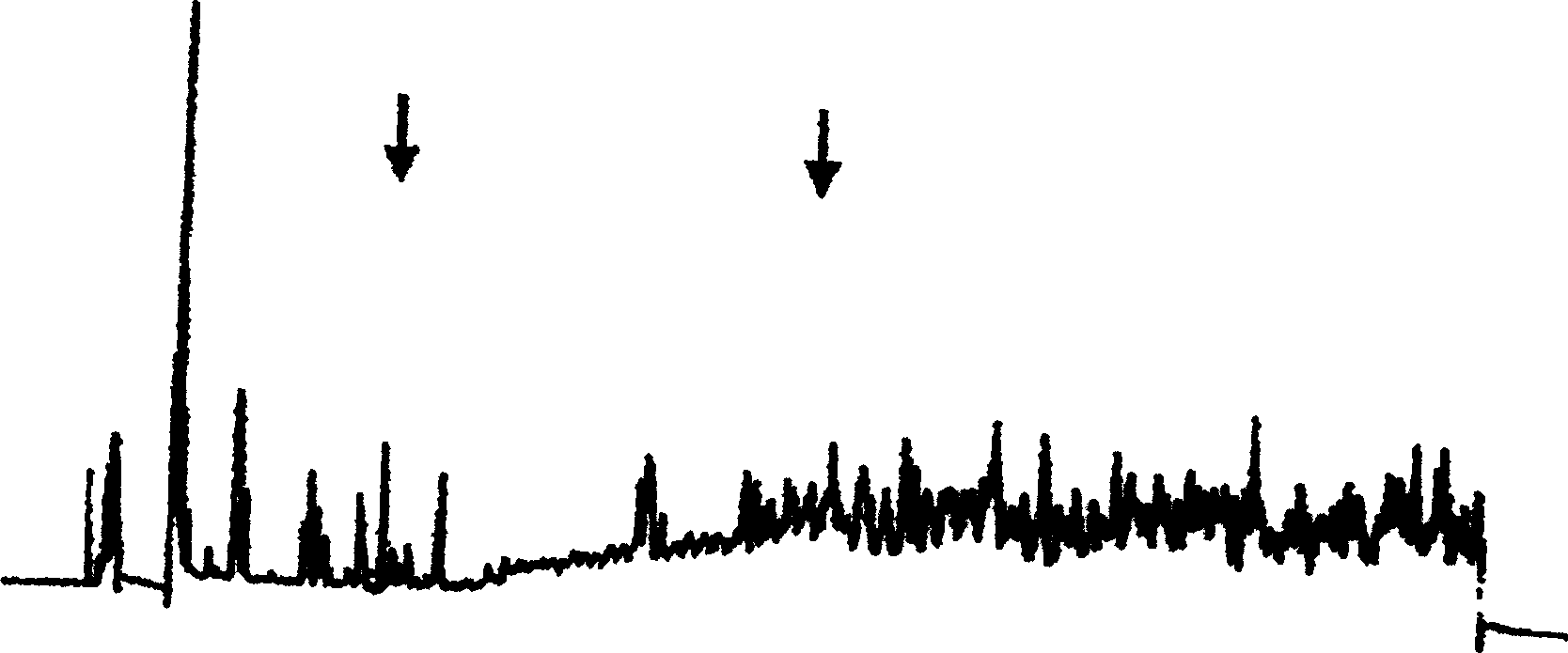
Image
Examples
Embodiment 1
[0125] composition example
[0126] Exemplary Composition A was prepared as follows. by dissolving 0.4 parts prostaglandin E in 5 parts ethanol 1 (Alprostadil USP) to obtain part A. Then, mix 5 parts of dodecyl-(N,N-dimethylamino)-propionate into alcohol-prostaglandin E 1 solution, followed by adding 5 parts of ethyl laurate.
[0127] Part B begins by preparing the water / buffer at pH 5.5. Water / buffer was prepared by adding sufficient potassium monohydrogen phosphate to pure water to obtain a 0.1M solution. The pH of the water / buffer was adjusted to 5.5 with strong base (1N sodium hydroxide) and strong acid (1N phosphoric acid). Buffer is about 80 parts of the total composition. All parts are by weight.
[0128] Add 0.5 parts of ethyl laurate to the buffer. Then, locust bean gum (in powder form) was dispersed in the buffer and homogenized with a stirring device. Table 1 is a list of ingredients. The resulting composition is an easy-to-apply semi-solid for application...
Embodiment 2
[0141] Example Compositions for Healthy Volunteers
[0142]A semisolid prostaglandin composition, Composition H, was administered to healthy volunteers in accordance with the methods of the present invention. It was found that a composition containing an amount of a semisolid prostaglandin component placed in the scaphoid fossa resulted in increased blood flow to the glans and promoted penile swelling. In a preferred embodiment, use by healthy individuals results in swelling of the glans, preferably penile erection. The study was conducted on 8 healthy volunteers without erectile dysfunction.
[0143] Table 2 summarizes some properties related to the study group. Eight healthy volunteers without ED were aged from 27 to 64 years (41.1±11.9, mean±standard deviation). Table 2 gives additional treatment outcome information for each healthy volunteer.
[0144] Evaluation of treatment is based on individual condition evaluation. Briefly, when the composition is administered, ba...
Embodiment 3
[0163] EXAMPLE COMPOSITIONS FOR ED PATIENTS
[0164] A semisolid prostaglandin composition, Composition H, was used in the treatment of ED in a group of patients using the method of the present invention. Placement of a composition comprising an amount of a semi-solid prostate component in the scaphoid fossa results in increased blood flow to the glans and promotes penile swelling. In preferred embodiments, the treatment results in swelling, preferably an erection. The study was performed on 13 ED patients.
[0165] The relevant characteristics of the studied groups are summarized in Table 3. Thirteen ED patients aged 33 to 68 years (51.0±10.3, mean±standard deviation) were screened and treated with an oral dose of 50 mg of sildenafil citrate (Viagra TM ) to achieve a full erection. The severity of erectile dysfunction is evaluated by the 5-item International Index of Erectile Function (IIEF-5). A low score of 5-item questionnaire within 0-25 indicates severe erectile dysf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com