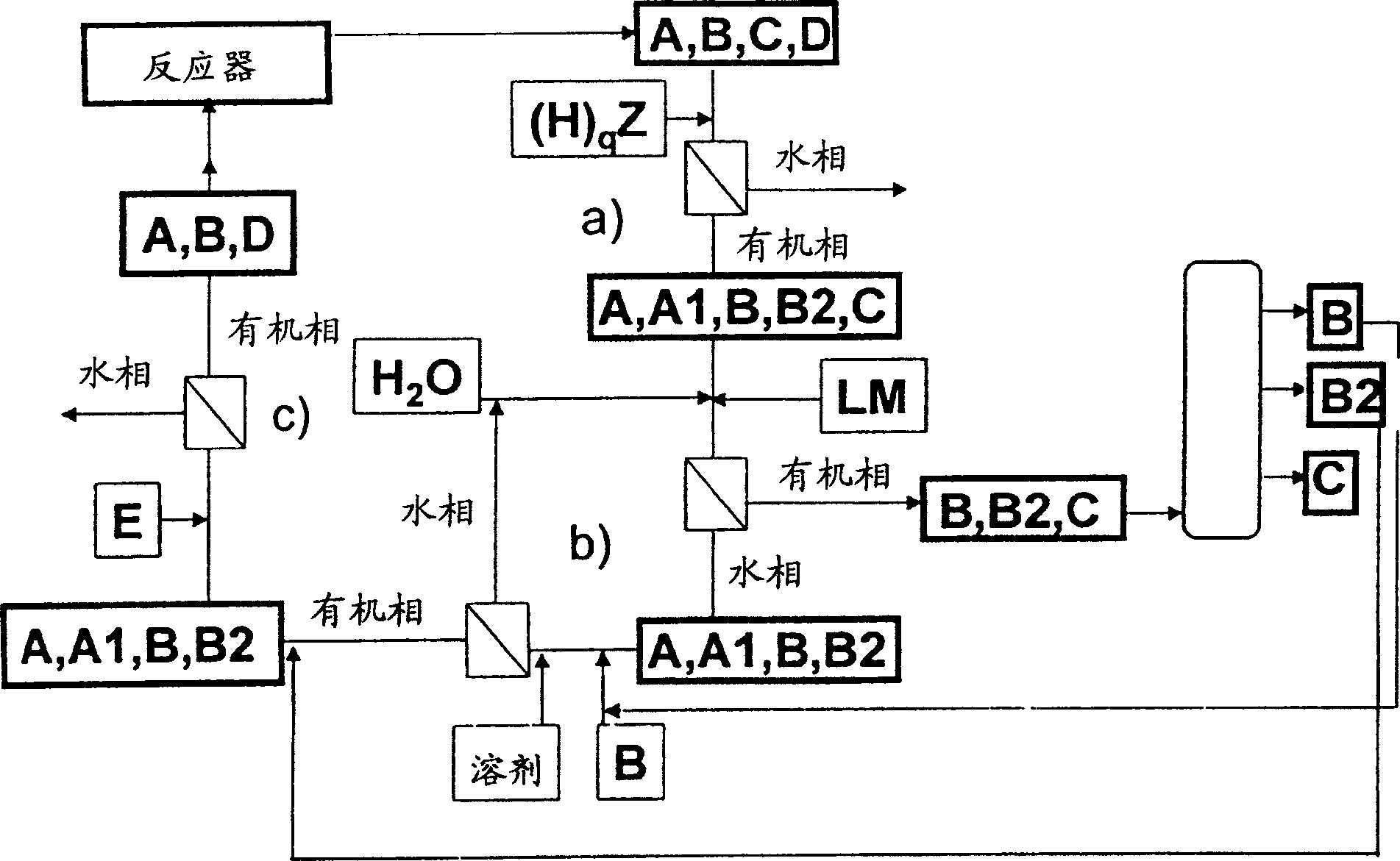
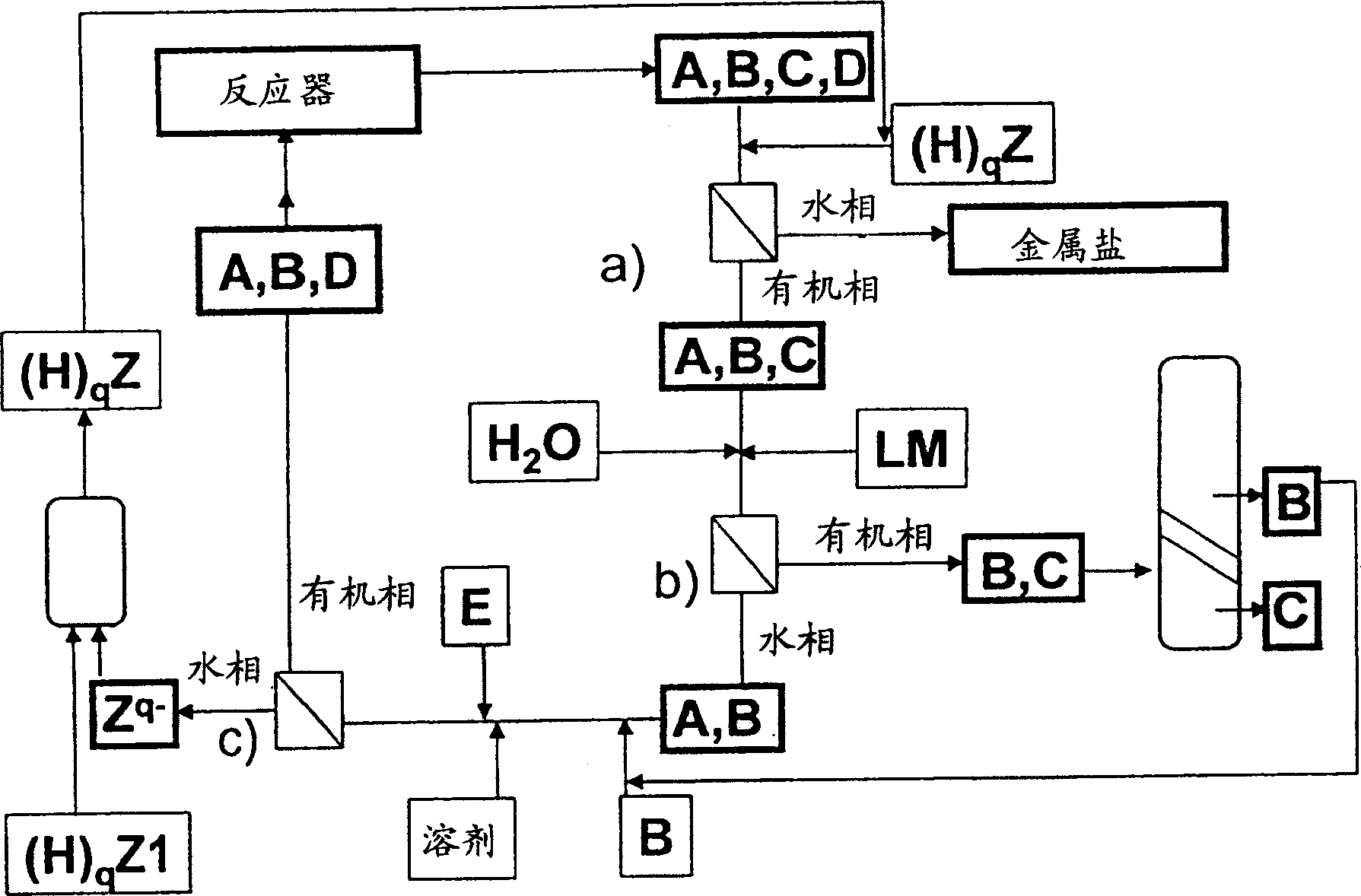
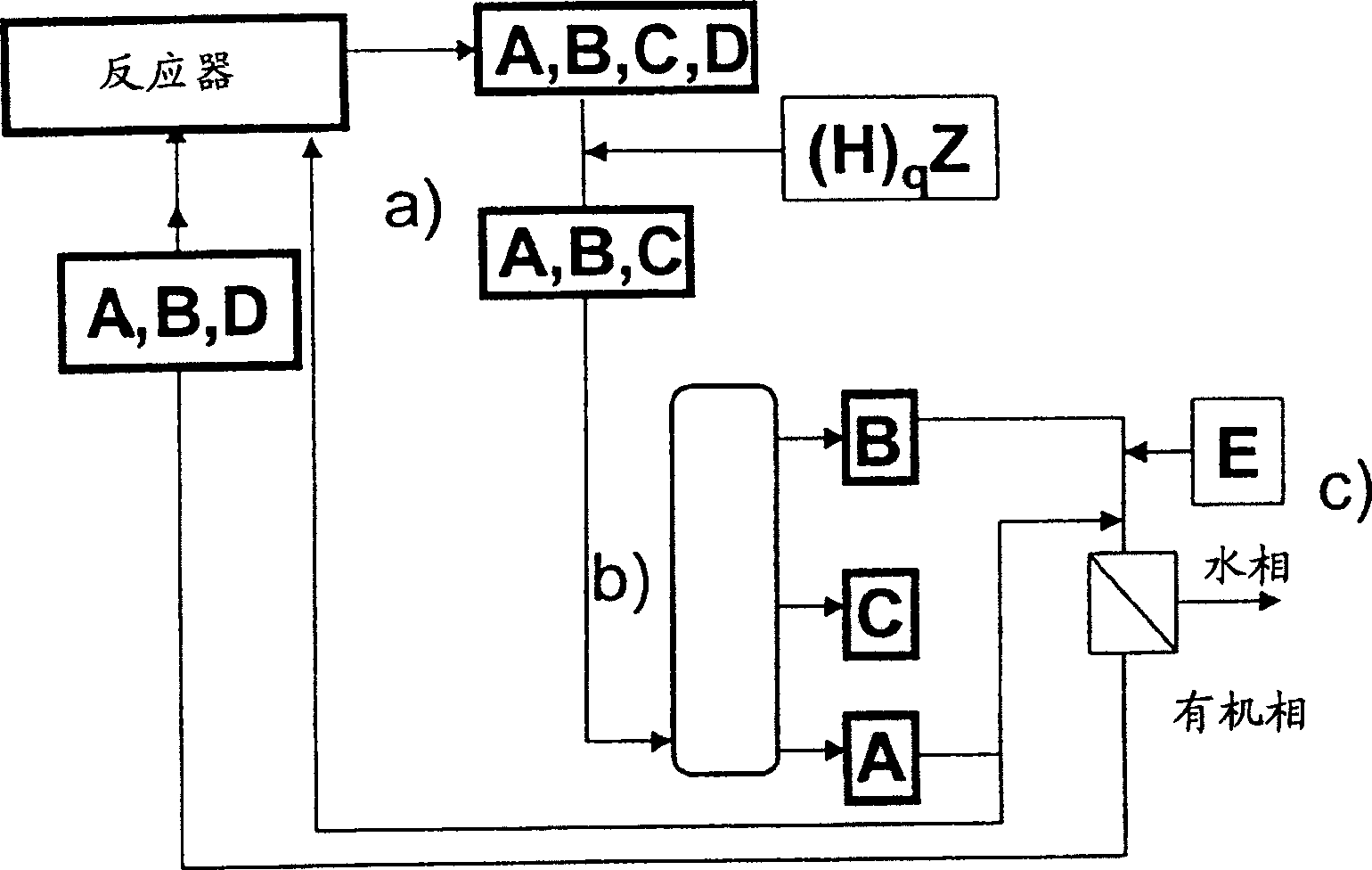
Method for separating reaction mixtures and recycling of quaternary salts and bases
A technology of reaction mixtures and mixtures, applied in chemical instruments and methods, preparation of organic compounds, catalytic reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] 5432 g of the reaction solution from the oxidative direct carbonylation process were extracted at 80°C with 33 ml of a 48% HBr solution in 543 g of water. 556.2 g of aqueous extract are obtained. Table 1 gives the composition of the solution after the single-step extraction.
[0140] mn
Embodiment 2
[0142] 200 g of 4% by weight tetrabutylammonium bromide, 4% by weight tetrabutylammonium phenate, 6% by weight phenol, 20 A solution of wt % DPC and 66 wt % chlorobenzene was used for extraction. The aqueous phases were then combined. Table 2 gives the composition of the solution.
Embodiment 3
[0147] A mixture of 50 g tetraphenylphosphonium bromide, 60 g chlorobenzene, 40 g phenol and 100 g diphenyl carbonate was distilled on a 5 cm high column packed with Rasching rings. Initially, the system was operated at a vacuum of 200 mbar until the temperature of the bottom of the column rose to about 180°C, then the vacuum was reduced to about 20 mbar and distillation was continued until the maximum temperature of the bottom of the column was about 200°C. The total amount of compound found in the distillate was calculated by adding the compositions of the fractions. 69.1 g of material from the reaction remained at the bottom of the column. The results are shown in Table 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com