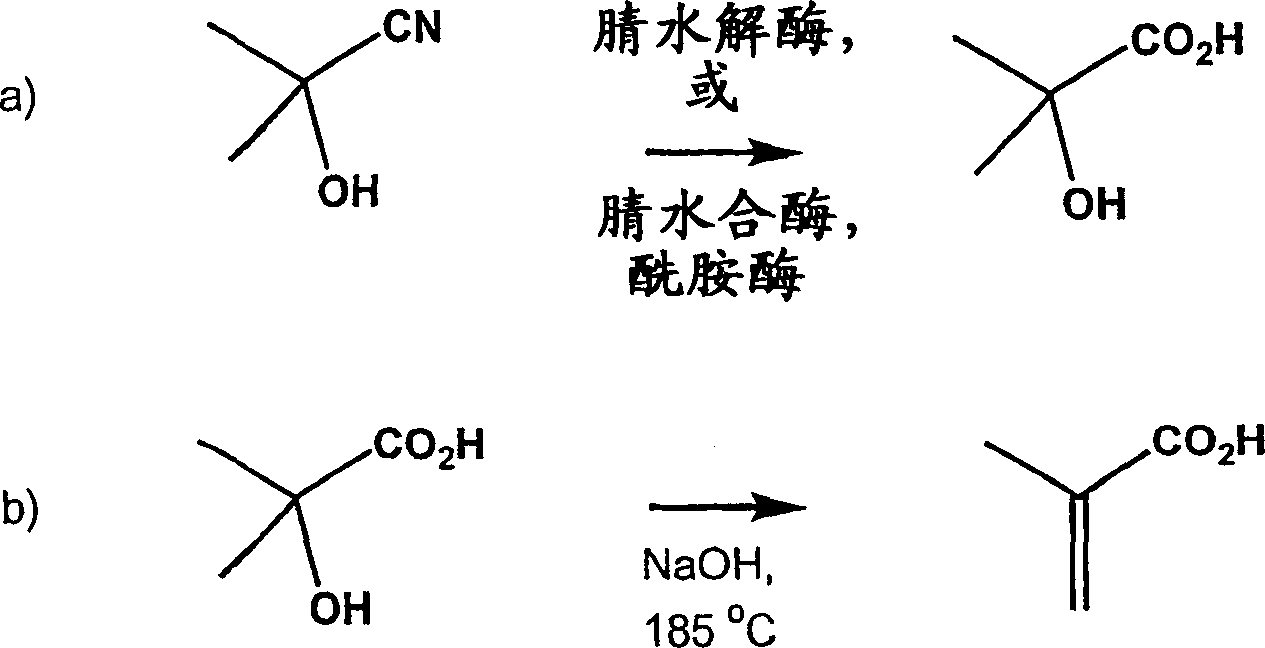
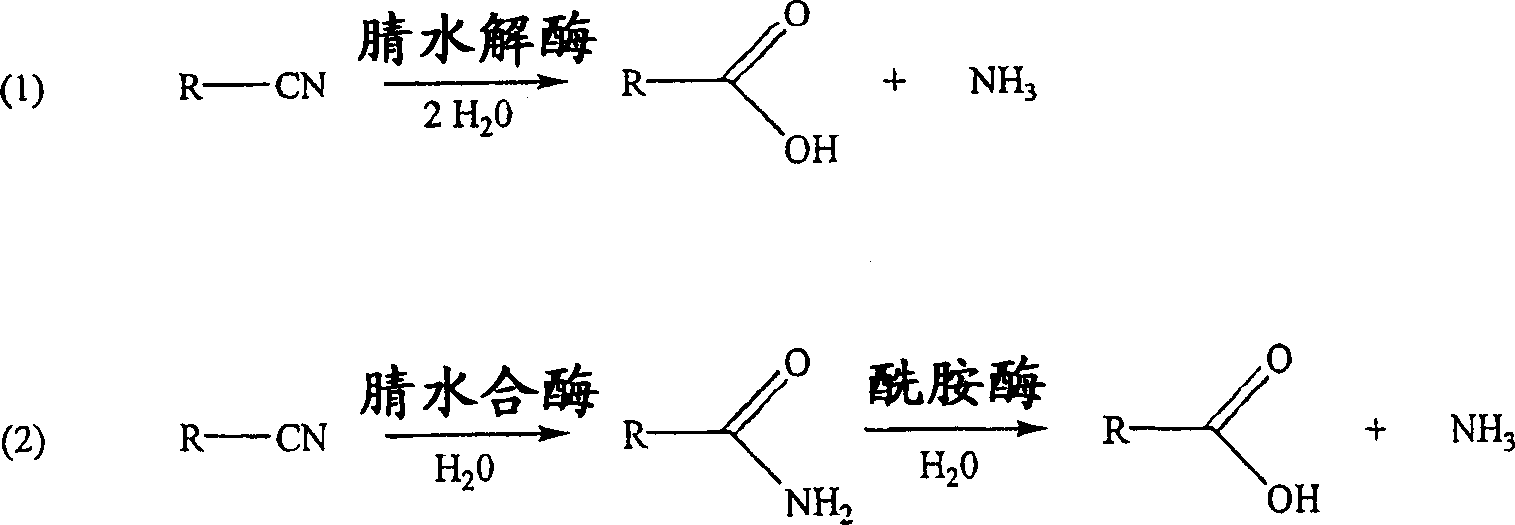
Method for producing 2-hydroxyisobutyric acid and methacrylic acid from acetone cyanohydrin
A technology of acetone cyanohydrin and hydroxyisobutyric acid, applied in the direction of chemical instruments and methods, microorganism-based methods, biochemical equipment and methods, etc., can solve the problems of lack, low waste production, enzyme inactivation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Hydrolysis of acetone cyanohydrin to 2-hydroxyl by Pseudomonas testosteroni 5-MGAM-4D cells Isobutyric acid
[0105] A suspension of 0.383g (pasty wet cells) of Pseudomonas testosterone 5-MGAM-4D cells (ATCC55744) in 5.56mL, 50mM potassium phosphate buffer (pH6.0) was placed in a 15mL polypropylene centrifuge tube, Then 51.0 mg of acetone cyanohydrin was added (the final concentration of acetone cyanohydrin in the suspension was 0.10 M) and the resulting suspension was mixed at 25° C. on a rotating platform. The sample for analysis (0.180 mL) was mixed with 0.020 mL of 1.0 M propionic acid (HPLC external standard), centrifuged, and the supernatant was analyzed by HPLC for acetone cyanohydrin, acetone and 2-hydroxyisobutyric acid. After 4 h, the yields of 2-hydroxyisobutyric acid, 2-hydroxyisobutyramide and acetone were 73.5%, 0% and 20.4%, respectively, and no acetone cyanohydrin remained.
Embodiment 2
[0107] Hydrolysis of acetone cyanohydrin to 2-hydroxyisobutyric acid by Pseudomonas testosteroni 22-1
[0108]A suspension of 0.343 g (pasty wet cells) of Pseudomonas testosteroni 22-1 cells (ATCC PTA-1853) in 5.61 mL, 50 mM potassium phosphate buffer (pH 6.0) was placed in a 15 mL polypropylene centrifuge tube , and then add 51.0 mg of acetone cyanohydrin (the final concentration of acetone cyanohydrin in the suspension is 0.10 M), and mix the resulting suspension at 25° C. on a rotating platform. The sample for analysis (0.180 mL) was mixed with 0.020 mL of 1.0 M propionic acid (HPLC external standard), centrifuged, and the supernatant was analyzed by HPLC for acetone cyanohydrin, acetone and 2-hydroxyisobutyric acid. After 21 hours, the yields of 2-hydroxyisobutyric acid, 2-hydroxyisobutyramide and acetone were 40.4%, 0% and 34.5%, respectively, and the remaining acetone cyanohydrin was 18.4%.
Embodiment 3
[0110] Conversion of acetone cyanohydrin to 2-hydroxyisobutyric acid by Pseudomonas facilis 72W 0.36g (paste wet cells) Pseudomonas facilis 72W cells (ATCC 55746) in 5.58 mL of the suspension in 100 mM potassium phosphate buffer (pH 6.0) was placed in a 15 mL polypropylene centrifuge tube containing 51.0 mg of acetone cyanohydrin (the final concentration of acetone cyanohydrin in the suspension was 0.10 M), and the resulting suspension was mixed on a rotating platform at 25°C. The sample for analysis (0.180 mL) was mixed with 0.020 mL of 1.0 M propionic acid (HPLC external standard), centrifuged, and the supernatant was analyzed by HPLC for acetone cyanohydrin, acetone, 2-hydroxyisobutyric acid and 2-hydroxy isobutyramide. After 22 hours, the yields of 2-hydroxyisobutyric acid, 2-hydroxyisobutyramide and acetone were 22.0%, 0% and 70.2%, respectively, and the remaining acetone cyanohydrin was 2.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com