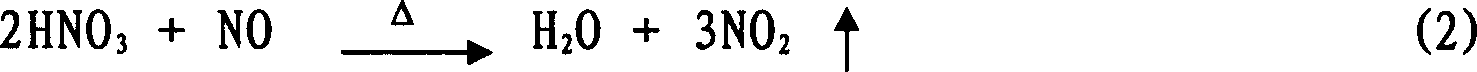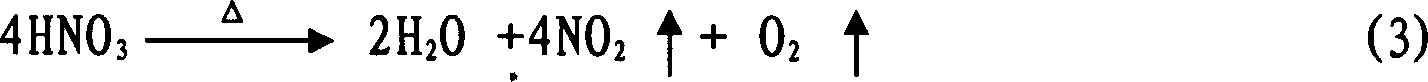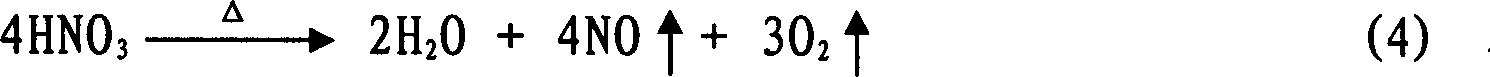Method for preparing H2SnO3 (tin dioxide) powder
A technology of tin dioxide and a manufacturing method, applied in the directions of tin oxide, chemical instruments and methods, tin compounds, etc., can solve the problems of unrecoverable nitrogen oxides, high environmental protection treatment costs, large consumption of nitric acid, etc. Promotion and application prospects, high tin conversion rate, and the effect of shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 28 liters of water, 2.5Kg tin flower, and 4.78 liters of 39.9% industrial nitric acid in a 50 liter pressurized reactor, heat and control it at 125±5°C, and react at a pressure of 0.9MPa for 6 hours. After the reaction was finished, the liquid and solid were separated to obtain 28 liters of the reaction raffinate and the intermediate product metastannic acid. The composition of the reaction raffinate is HNO 3 56.74g / L, Sn0.000048g / L, Fe0.00048g / L, Pb0.016g / L. Reaction raffinate replenishes 1.72 liters of industrial nitric acid and returns to the pressurized reactor, and the mass ratio of the acid tin to be controlled is 0.952 (same as the reaction conditions), and 2.5Kg of tin flower is added in addition, and reacts under the above-mentioned reaction conditions, and the residual acid after the reaction is re- Supplementary acid returns to the reaction, and so on. The intermediate product metastannic acid that reaction produces is processed by existing production m...
Embodiment 2
[0029] at 2m 3 Add water 1.2m into the pressurized reactor 3 , tin powder 110Kg, industrial nitric acid (including HNO 3 39.9%) 270 liters, heated to 140°C, and reacted for 5 hours under the pressure of 0.7MPa. After the reaction, the liquid and solid were separated to obtain a reaction residue of 1.15m 3 And the intermediate product metastannic acid, the reaction raffinate composition is HNO 3 62.17g / L, Sn0.000585g / L, Fe0.0016g / L, Pb0.009g / L. Add 130 liters of industrial nitric acid to the residual liquid and return to the pressurized reactor, control the mass ratio of acid tin to 1.34 (same as the above reaction), add 110Kg of tin powder, react under the above reaction conditions, and return the residual acid after the reaction to supplement the acid , and back and forth. The intermediate product metastannic acid produced by the reaction is processed according to the existing production method. After neutralization and washing, 123Kg of tin dioxide is obtained after dry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com