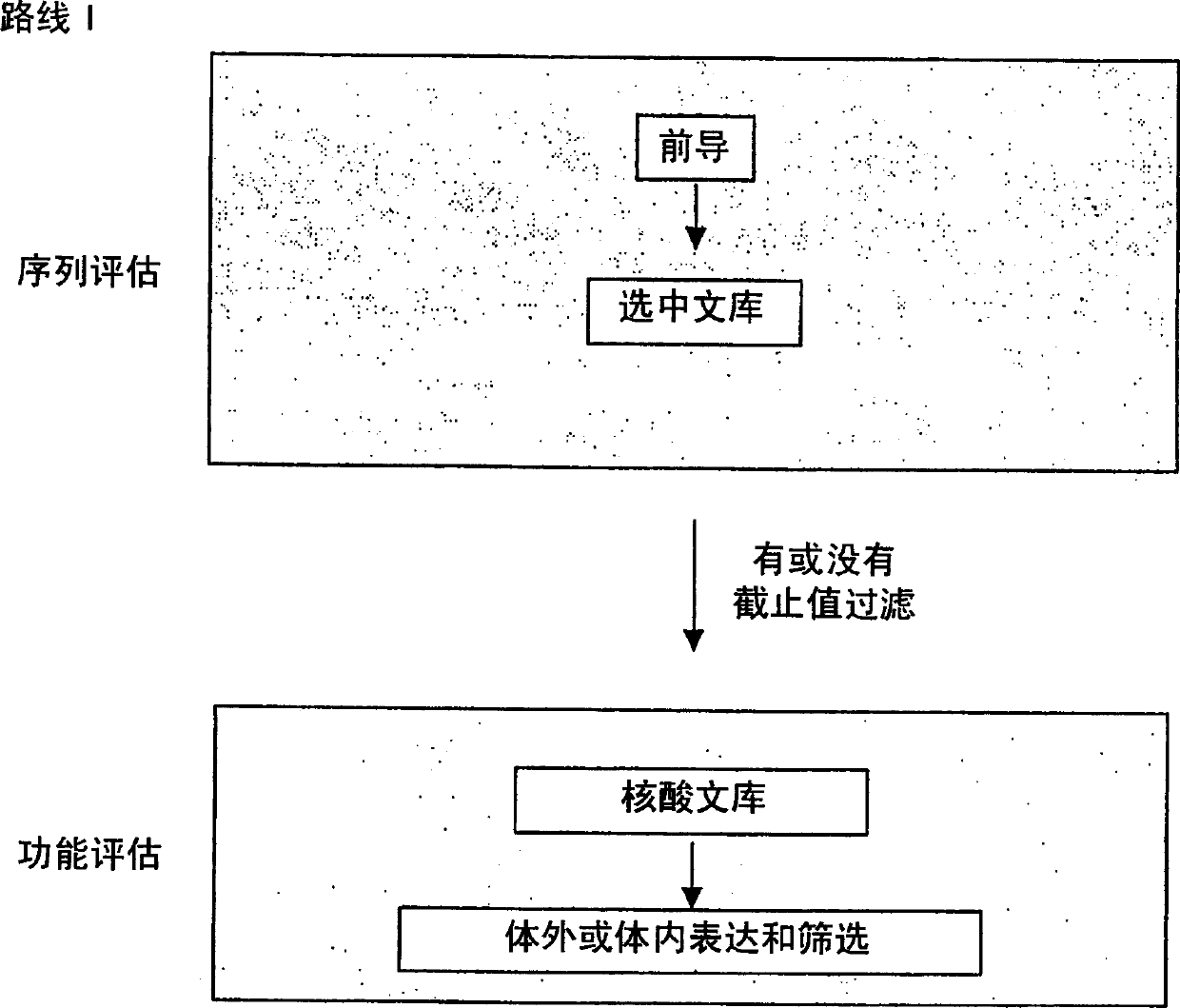
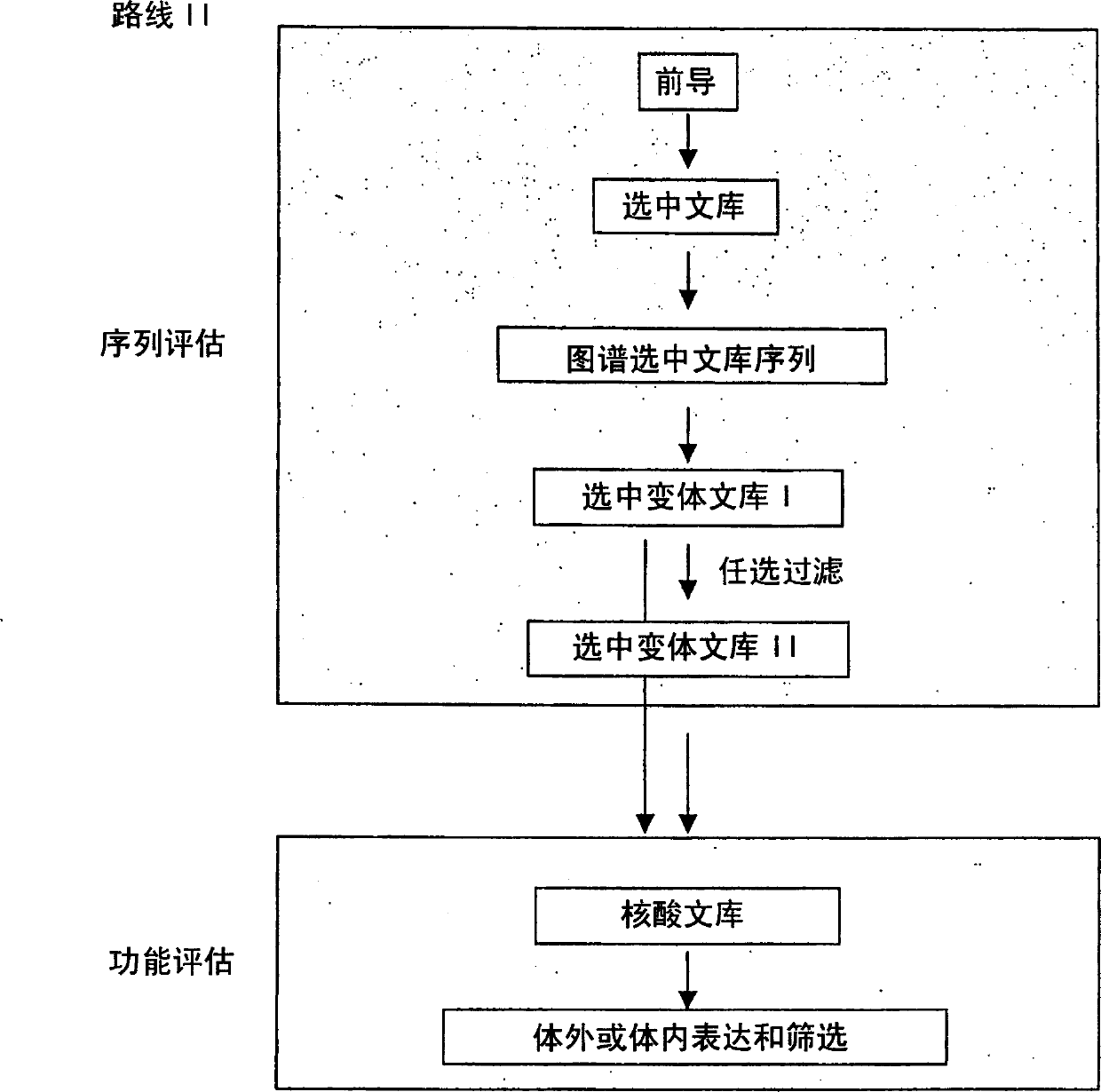
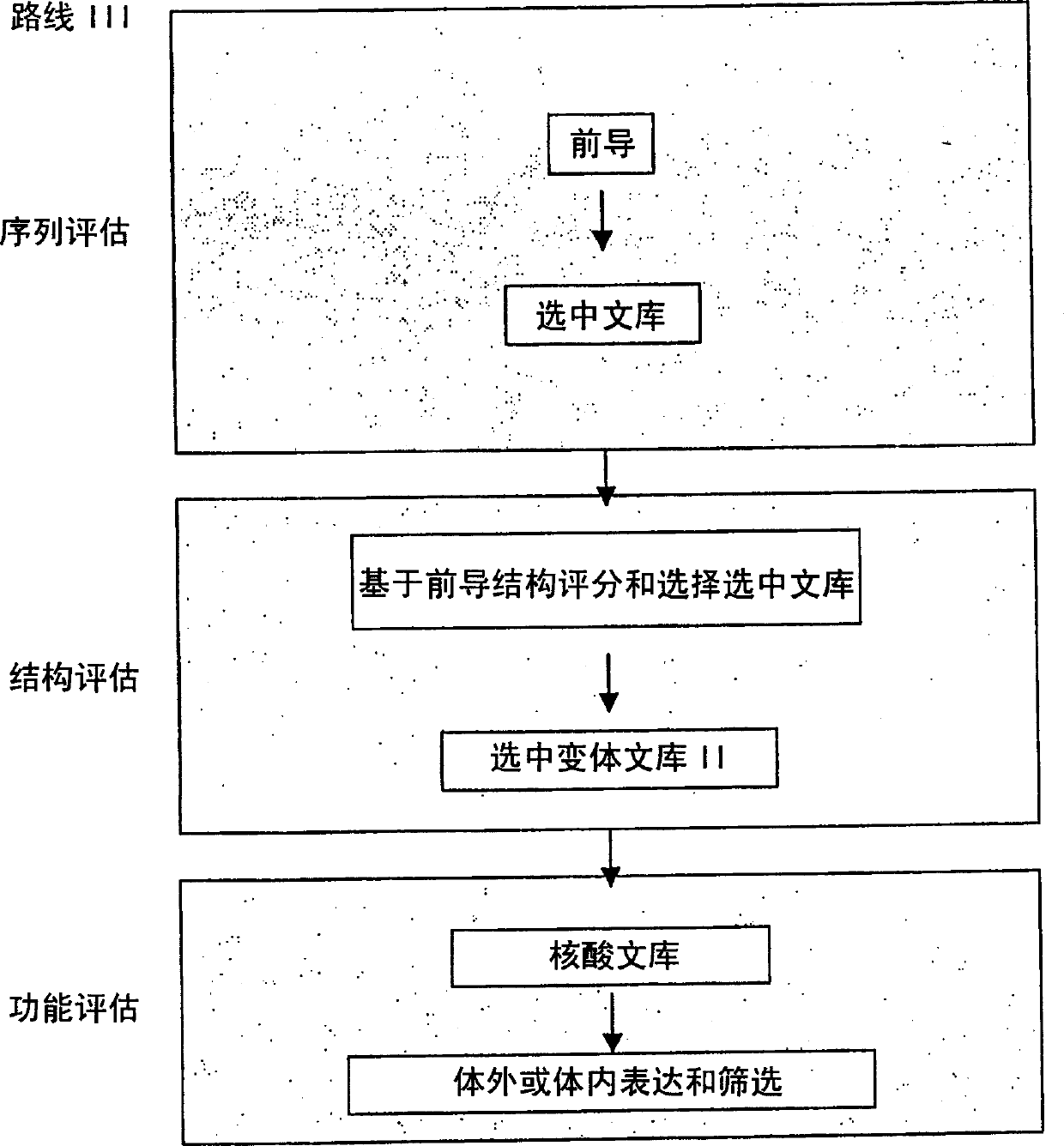
Generation and selection of protein library in silico
A protein sequence and library technology, applied in proteomics, computer combinatorial chemistry, anti-growth factor immunoglobulin, etc., can solve unpredictable and labor-intensive problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0816] The methods of the present invention are used to construct antibody libraries in silico. Vascular endothelial growth factor (VEGF) was chosen as the antigen for the proof-of-principle experiments of the invention to demonstrate the invention in antibody design. For VEGF and its receptors (Muller YA, Christinger HW, Keyt BA, de Vos AM (1997) Structure 5, 1325-1338; Wiesmann C, Fuh G, Christinger HW, Eigenbrot C, Wells JA, de Vos AM (1997) ) Cell 91, 695-704), the complex between VEGF and its humanized antibody (Muller YA, Christinger HW, Li B, Cunningham BC, Lowman HB, de Vos AM (1998) Structure 6, 1153-1167 , and the complex between VEGF and its mature antibody (Chen Y, Wiesmann C, Fuh G, Li B, Christinger HW, McKay P, de Vos AM (1999) J Mol Biol 293, 865-881), available Rich collection of sequence and structural information. These provide a good platform for testing the methods of the invention. By using the methods provided by the invention, by using the increased weal...
Embodiment 2
[0897] Example 2 Generation of Anti-VEGF Antibody Library for Framework Optimization
[0898] VEGF is a key angiogenic factor in development and is involved in the growth of solid tumors by stimulating endothelial cells. Murine monoclonal antibodies found to block VEGF-dependent cell proliferation and slow tumor growth in vivo (Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N (1993) Nature 362, 841-844) . After grafting the antigen-binding loop, the murine antibody was humanized using random mutagenesis at some key framework positions (Presta LG, Chen H, O'Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N( 1997) Cancer Res. 57, 4593-4599; Baca M, Presta LG, O'Connor SJ, Wells JA (1997) J Biol Chem 272, 10678-10684). Typically, after several rounds of site-directed mutagenesis and selection, humanized antibodies are generated by substituting non-human amino acids from the parental non-human antibody at certain pre-determined critical position...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com