Histone methyl transferase and its preparing method
A protein and protein polypeptide technology, applied in the field of molecular biology, can solve problems such as unclear functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
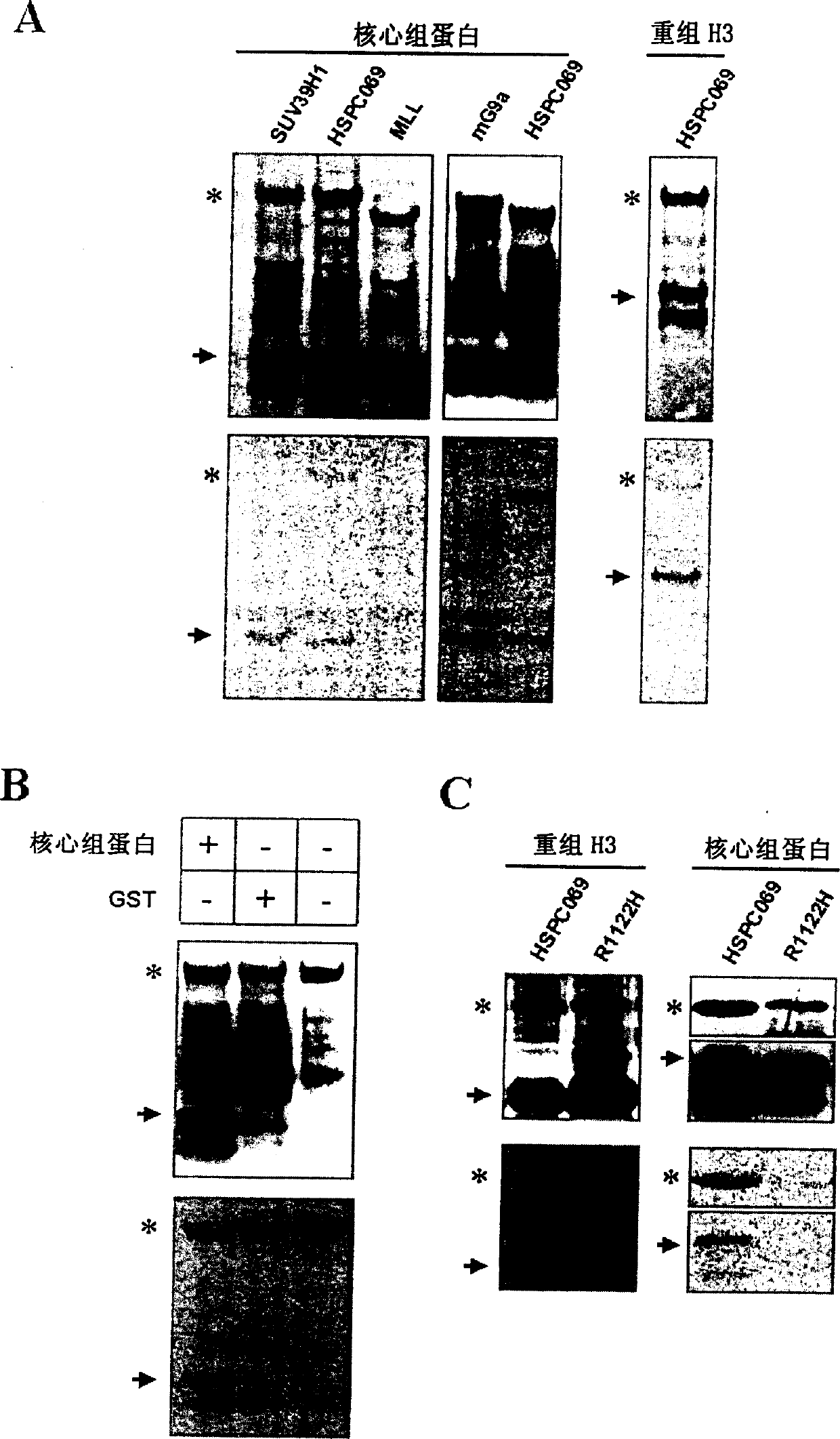
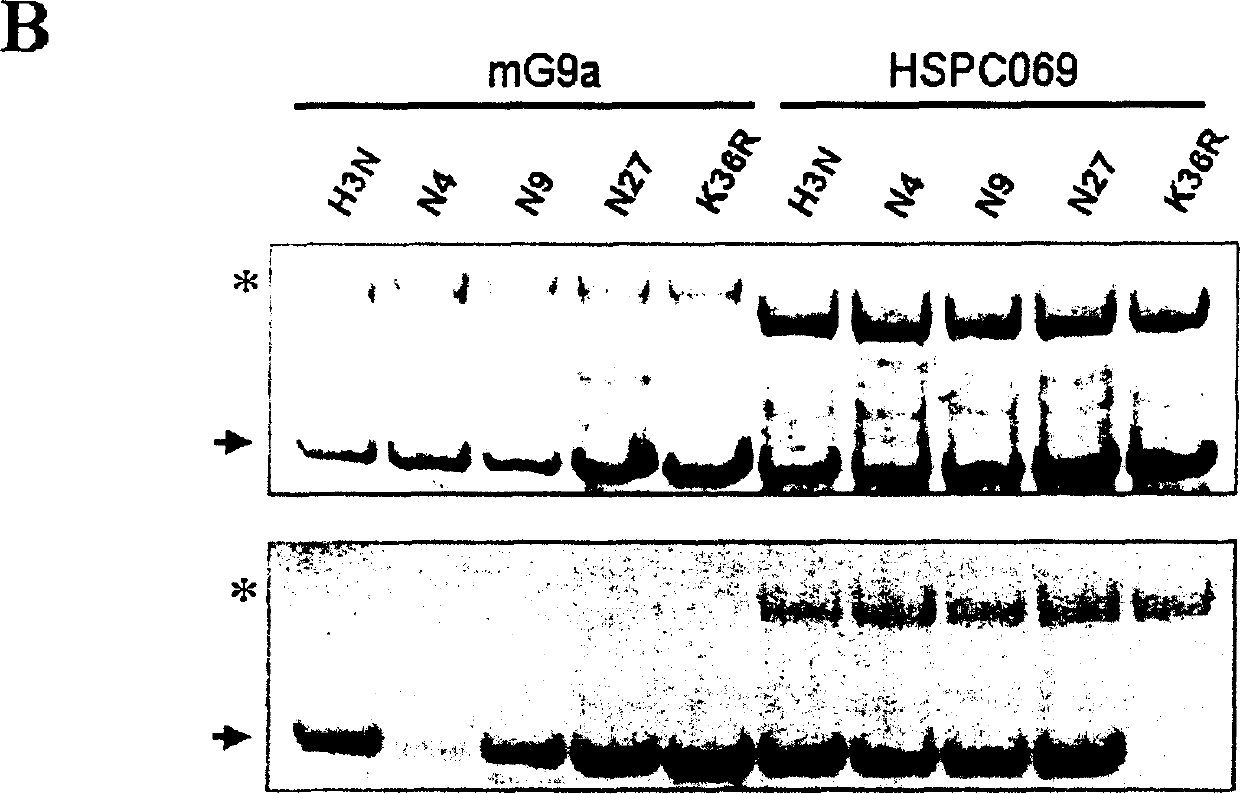
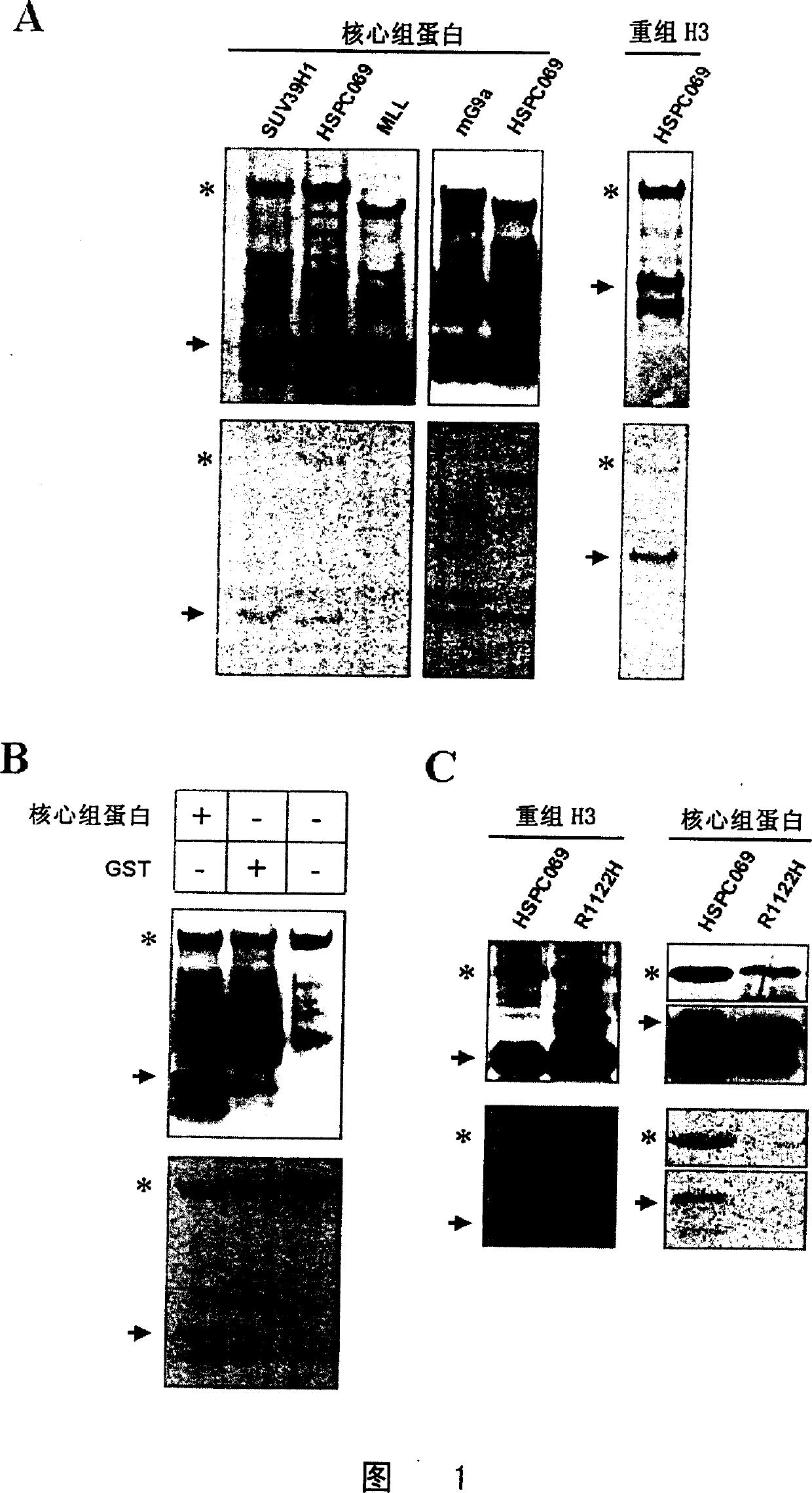
Image
Examples
Embodiment 1
[0094] Embodiment 1: Cloning of HSPC069SET protein cDNA
[0095] The cDNA fragment of HSPC069SET comes from a CD34-positive hematopoietic stem / progenitor cell cDNA library constructed by conventional methods (see Zhang et al., Genome Res .2000, 10:1546-6). PCR was performed using the cDNA library as a template and a pair of oligonucleotides as primers—upstream: ctcagatctaacagggacctaaggacatcatc (SEQ ID NO: 7) and downstream: cgcggtaccttattttcaatatattcacatatacatta (SEQ ID NO: 8). The amplified fragment was digested with BglII / KpnI, and connected into the pEGFP vector (Clontech Company) to obtain the HPC069SET-pEGFP plasmid, and the nucleotide sequence of the obtained HSPC069SET was identified by sequencing.
[0096] The full length of HSPC069SET cDNA is 6731bp (SEQ ID NO: 1), contains a complete open reading frame (position 72-3704), and encodes a polypeptide containing 1211 amino acid residues (SEQ ID NO: 2). Homology comparison shows that this amino acid sequence contains a...
Embodiment 2
[0102] Expression and purification of HSPC069SET protein in Escherichia coli
[0103] Using the extracted hematopoietic stem cell mRNA as a template, after reverse transcription, use a pair of oligonucleotides as primers-A: gcgtcgacgtgatggtgagcttcaggacaga (SEQ ID NO: 9) and B: aactgcagatgtgaggcagacaagtcattcca (SEQ ID NO: 10) to perform PCR . The amplified fragment was digested with restriction endonuclease SalI / PstI, and the product was ligated into pGBKT7 (purchased from Clontech Company) to obtain the HPC069SET-pGBKT7 plasmid, and the obtained cDNA was identified by sequencing.
[0104] Then, HPC069SET-pGBKT7 was digested with restriction endonuclease EcoRI, and then ligated into pGEX-5X1 vector (Amersham Biosciences) to obtain HSPC069SET-pGEX-5X1 plasmid. Sequencing confirmed that the cDNA fragment of HSPC069SET was correctly inserted into the vector. Escherichia coli BL21 strain was transformed with this plasmid. Positive transformants were cultured overnight in LB medi...
Embodiment 3
[0107] Construction of mutants and acquisition of mutant proteins
[0108] Using the conventional bridge PCR method, on the basis of the HSPC069SET-pGEX-5X1 plasmid prepared in Example 2, the 1122th arginine in the SET domain of the HSPC069SET protein was mutated to histidine (i.e. SEQ ID NO: 449th out of 4). By the same method as in Example 2, the mutated HSPC069SETMp protein was obtained with the mutant plasmid,
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com