Application of inhibitor of histone methyltransferase EZH2 to preparing medicine for controlling peritoneum fibrosis after peritoneal dialysis
A methyltransferase, peritoneal dialysis technology, applied in the direction of drug combination, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve the peritoneal function and histopathological changes, limit the application value of CAPD, reduce the ultrafiltration of peritoneal dialysis Volume and other issues, to achieve the effect of reducing peritoneal pathological changes, significant technological progress, and reducing angiogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 Materials and methods
[0022] 1) Reagent consumables
[0023] p-STAT3, STAT3, p-Smad3, Smad3, p-NF-κBp65, NF-κBp65, p-EGFR, EGFR, EZH2, α-Tubulin, E-cadherin, H3K27me3, Notch1, Jagged-1, p-Src, Src, MMP2, and MMP9 antibodies were all purchased from cellsignaling Technology. Collagen I (A2), TGF-βRI, CD68, CD3, EGFR, VEGF, CD31 and GAPDH antibodies were purchased from Santa Cruz Company. TNF-α, IL-1β, MCP-1, IL-6, VEGF, CA125 and TGF-β1 (ELISA) kits were purchased from Roche Life Science. 3-DZNeP was purchased from Selleckchem. α-SMA antibody and other reagents were purchased from Sigma.
[0024] 2) Mouse peritoneal fibrosis model and experimental grouping
[0025] The mouse peritoneal fibrosis model was established by intraperitoneal injection of 4.25% high glucose peritoneal dialysis fluid. All animal experiments complied with the Chinese regulations on the management and use of laboratory animals.
[0026] ①28-day 4.25% high-glucose peritoneal dia...
Embodiment 2
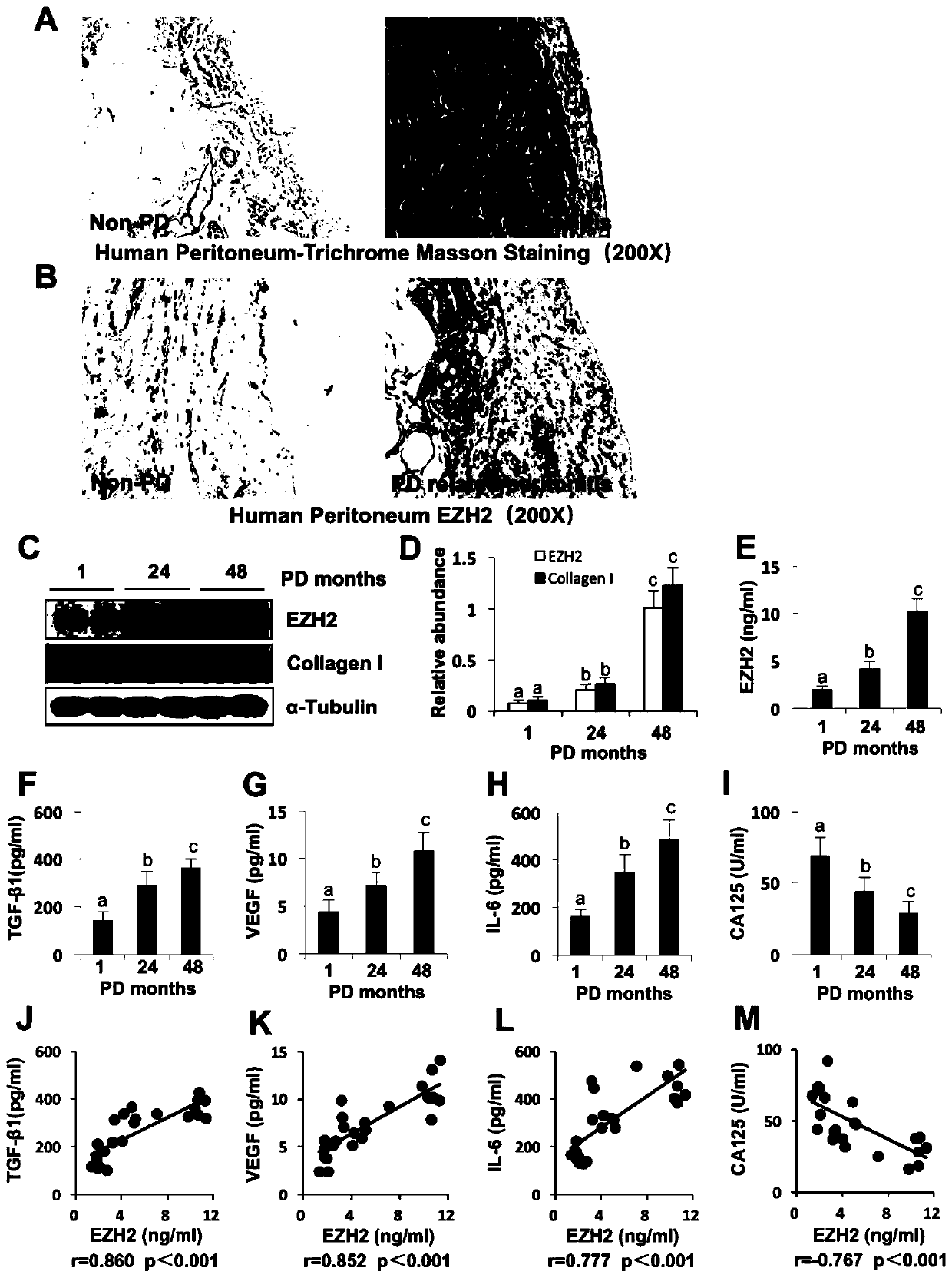
[0053] Example 2 EZH2 is highly expressed in peritoneal tissue of patients with peritonitis associated with peritoneal dialysis, and is positively correlated with TGF-β1, VEGF and IL-6 in peritoneal dialysis effluent, and negatively correlated with CA125
[0054] In the peritoneal tissue staining of non-peritoneal dialysis patients and peritonitis-related peritonitis patients, it was found that the positive area of Masson staining in peritonitis patients was significantly higher than that of non-peritoneal dialysis patients, and the expression of EZH2 was also significantly increased ( figure 1 A and 1B). The peritoneal dialysis effluents of patients with peritoneal dialysis at 1 month, 24 months, and 48 months were collected for Western blot detection, and it was found that the longer the peritoneal dialysis age, the higher the expression of EZH2 and Collagen I ( figure 1 C and 1D). ELISA detected the contents of various indicators in the peritoneal dialysis effluent of pa...
Embodiment 3
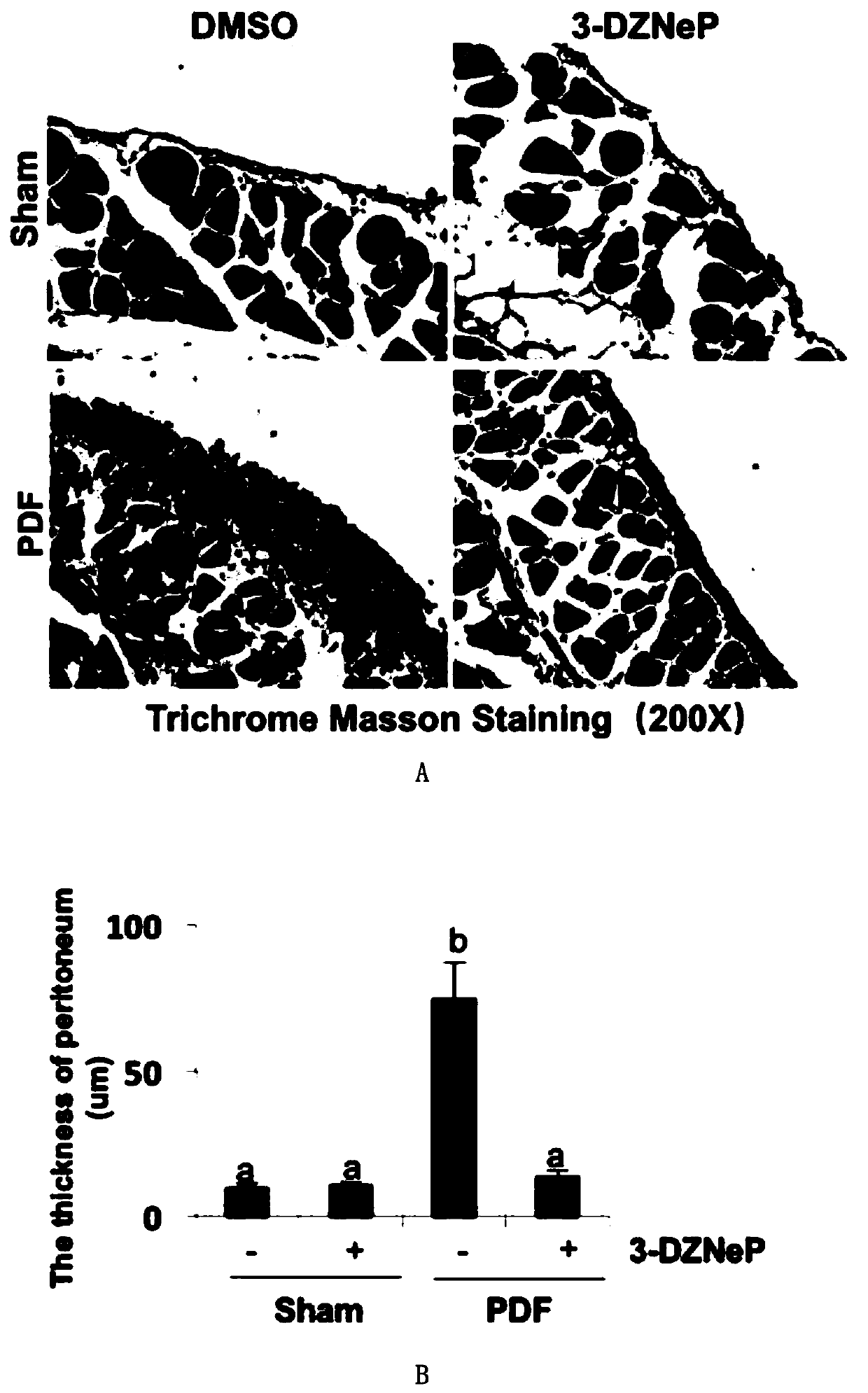
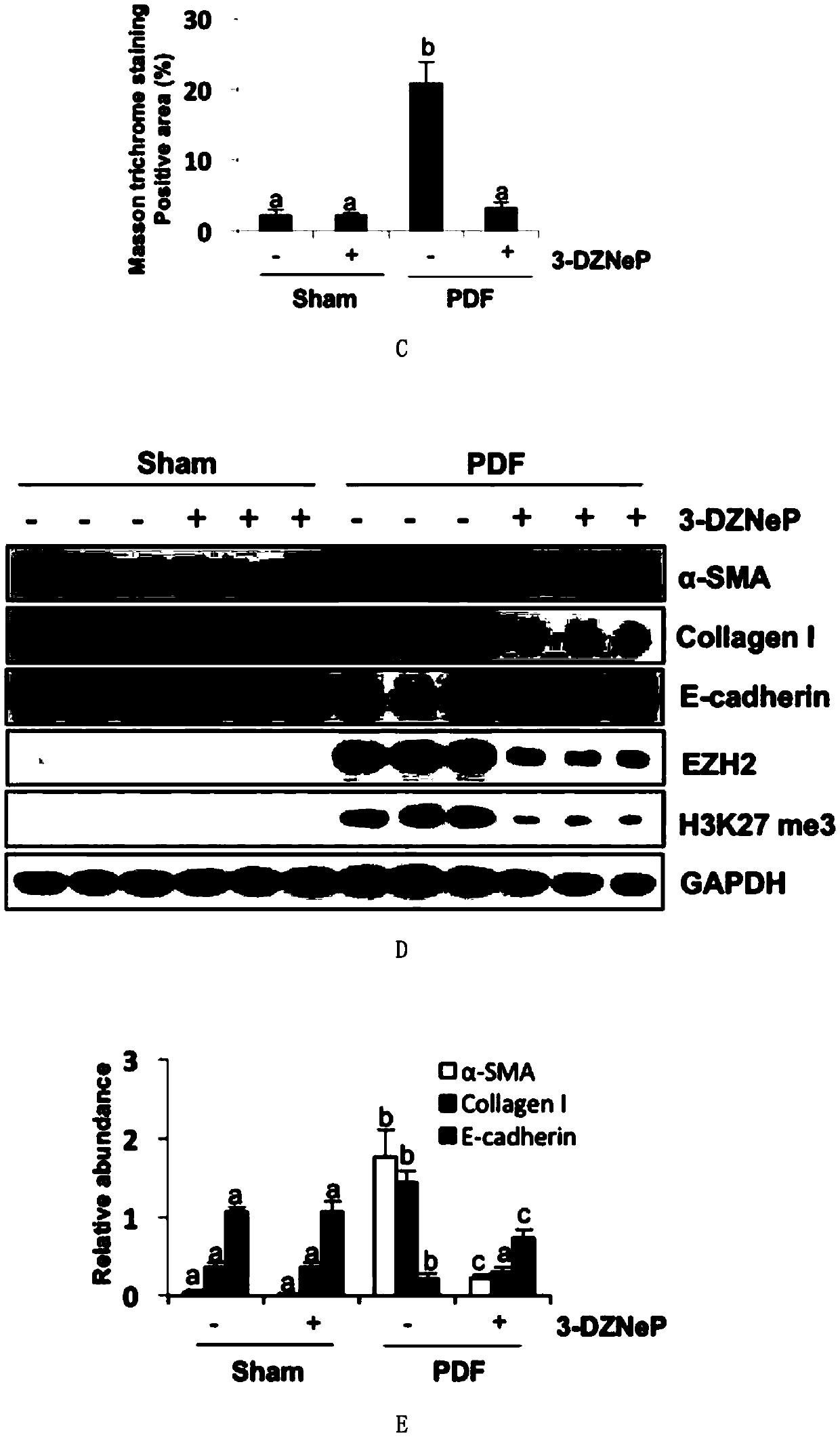
[0055] Example 3 3-DZNeP inhibits the occurrence of peritoneal fibrosis in mice induced by high glucose peritoneal dialysis fluid (PDF)
[0056] In the animal model, the present invention injects 3 ml of 4.25% high-glucose peritoneal dialysis fluid into mice intraperitoneally, and successfully establishes an animal model of peritoneal fibrosis after 28 days. Masson staining shows that the subperitoneal area of the model is obviously thickened. After daily injection of 3-DZNeP in the treatment group, the thickness of the peritoneum and the positive area of Masson staining could be significantly reduced ( figure 2 A-2C), showing that 3-DZNeP plays an important role in mediating peritoneal fibrosis. Western blotting results showed that EZH2 and H3K27me3 were highly expressed in the peritoneal tissue of mice with peritoneal fibrosis, and the expression of both could be downregulated after using 3-DZNeP ( figure 2 D and 2F). 3-DZNeP can also down-regulate the high expressio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com