Polyethylene glycol-modified thymus peptide 1 derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
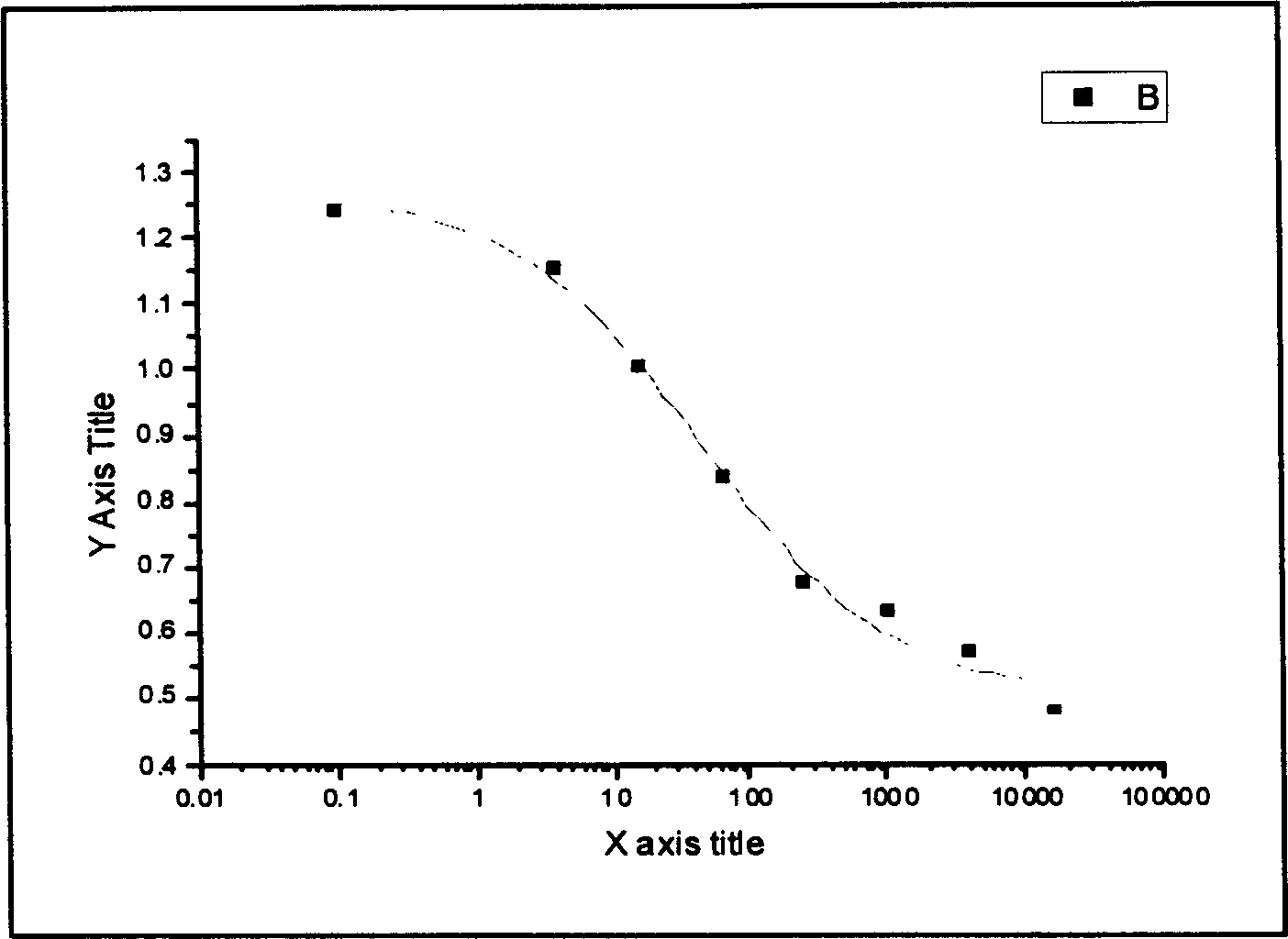
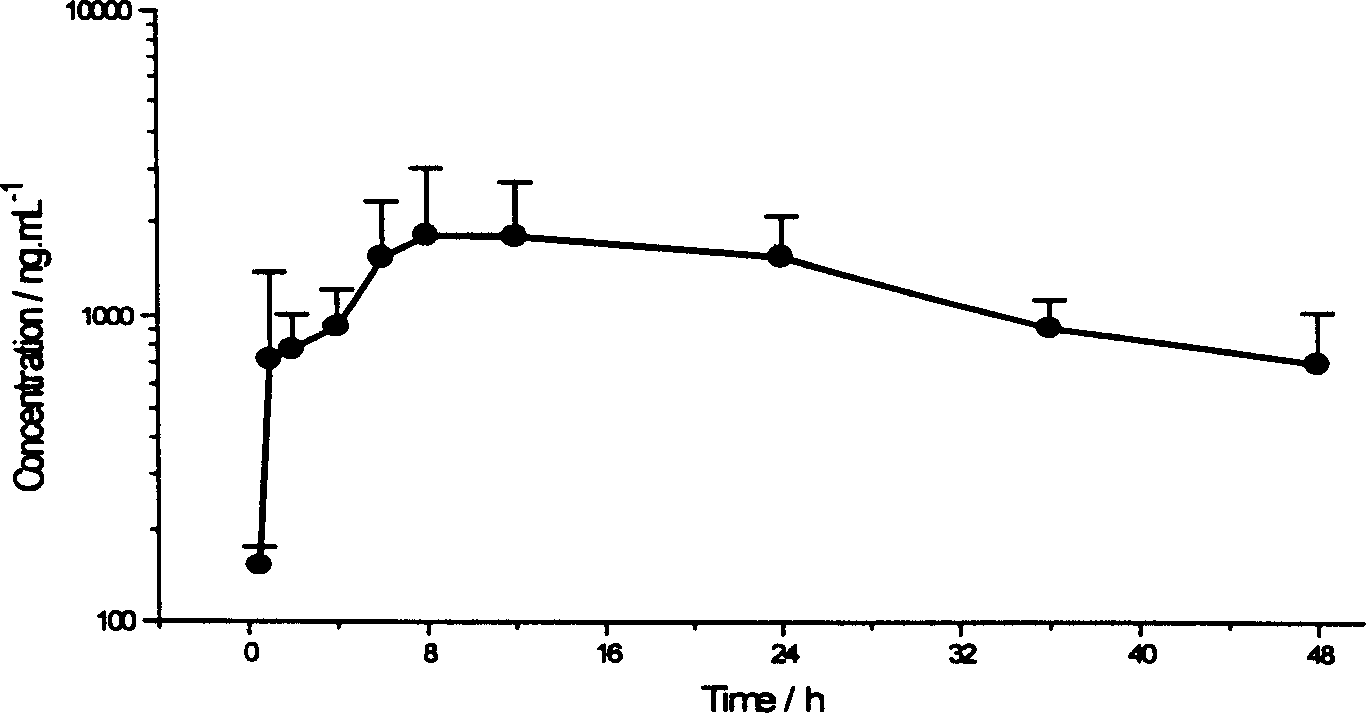
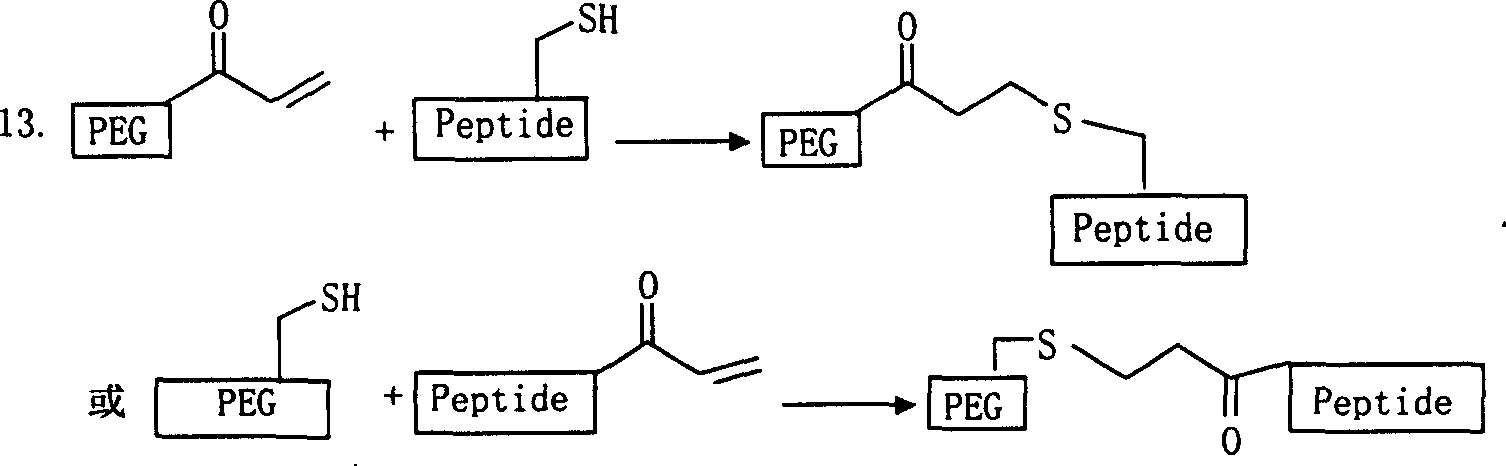
Image
Examples
Embodiment 1
[0098] Ac-Ser-Asp-Ala-Ala-Val-Asp-Thr-Ser-Ser-Glu-Ile-Thr-Thr-Lys-Asp-Leu-Lys-Glu-Lys-Lys-Glu-Val-Val-Glu- Preparation of Glu-Ala-Glu-Asn-Glu-Cys-PEG40K
[0099] Step 1: Precursor for polyethylene glycol modification—C-terminal cysteine thymosin 1 derivative prepared by solid-phase chemical synthesis:
[0100] (1) Amino acid monomers used
[0101] Fmoc-Ala-OH Fmoc-Lys(Boc)-OH
[0102] Fmoc-Asn(Trt)-OH Fmoc-Ser(tBu)-OH
[0103] Fmoc-Asp(OtBu)-OH Fmoc-Thr(tBu)-OH
[0104] Fmoc-Cys(Trt)-OH Fmoc-Val-OH
[0105] Fmoc-Glu(OtBu)-OH Fmoc-Ile-OH
[0106] Fmoc-Leu-OH
[0107] The abbreviation in the above formula means:
[0108] Fmoc: 9-fluorenylmethoxycarbonyl (9-fluorenylmethoxycarbonyl)
[0109] Boc: tert-butyloxycarbonyl (tert-butyloxycarbonyl)
[0110] Trt: Trityl (trityl)
[0111] OtBu: tert-butyl
[0112] tBu: tert-butyl
[0113] (2) The equipment and reagents used in the synthesis
[0114] Instruments: The synthesis of peptide sequences is carried out manually, an...
Embodiment 2
[0142] Ac-Ser-Asp-Ala-Ala-Val-Asp-Thr-Ser-Ser-Glu-Ile-Thr-Thr-Arg-Asp-Leu-Arg-Glu-Arg-Arg-Glu-Val-Val-Glu- Glu-Ala-Glu-Asn-Lys-PEG40K
[0143] Step 1: Solid-phase chemical synthesis to prepare precursor C-terminal lysine 14, 17, 19, 20, arginine-substituted thymosin 1 derivatives for polyethylene glycol modification:
[0144] For the solid-phase chemical synthesis method, see Example 1 for the solid-phase chemical preparation of the C-terminal cysteine thymosin 1 derivative. 10 g of Wang resin (0.6 mmol / g resin, 6 mmol) linked with Fmoc-Lys(Boc)-OH was used for synthesis. The synthesis follows the Fmoc amino acid and the synthesis cycle is repeated with the corresponding amino acid in each cycle step:
[0145] Fmoc-Asn(Tre)-OH, Fmoc-Ala-OH, Fmoc-Glu(OtBu)-OH,
[0146] Fmoc-Glu(OtBu)-Ort,
[0147] Fmoc-Val-OH, Fmoc-Val-OH, Fmoc-Glu(OtBu),
[0148] Fmoc-Arg(Pbf)-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Glu(OtBu)-OH,
[0149] Fmoc-Arg(Pbf)-OH, Fmoc-Leu-OH, Fmoc-Asp(OtBu)-OH,
[0150] Fm...
Embodiment 3
[0158] Embodiment three drug efficacy experiment
[0159] Using Zidaxian as a positive control, the effect of the sample on the production of cytokines by T lymphocytes was detected to determine whether the sample has a regulatory effect on the immune activity of T lymphocytes and the strength of the effect.
[0160] [Experimental principle]
[0161] Among the lymphocytes cultured in vitro, T lymphocytes can activate T lymphocytes after being stimulated by mitotic proconcanavalin A (ConA, Concanavalin A), and produce cytokine synthesis, cytokine receptor expression, cell differentiation and cell proliferation. Changes in cell behavior. The production of cytokines and the proliferative response of cells are the reflection of the functional state and cell activation of immune cells. The detection method has the characteristics of reliability, good repeatability and simplicity, so it has been widely used to judge the influence of samples on the function of T lymphocytes through...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com