Method for preparing cefixime
A cefixime and compound technology, applied in the field of pharmaceutical synthesis, can solve the problems of difficult drying, poor sulfate purity, low yield and the like, and achieve the effects of light appearance, avoiding inconvenience and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
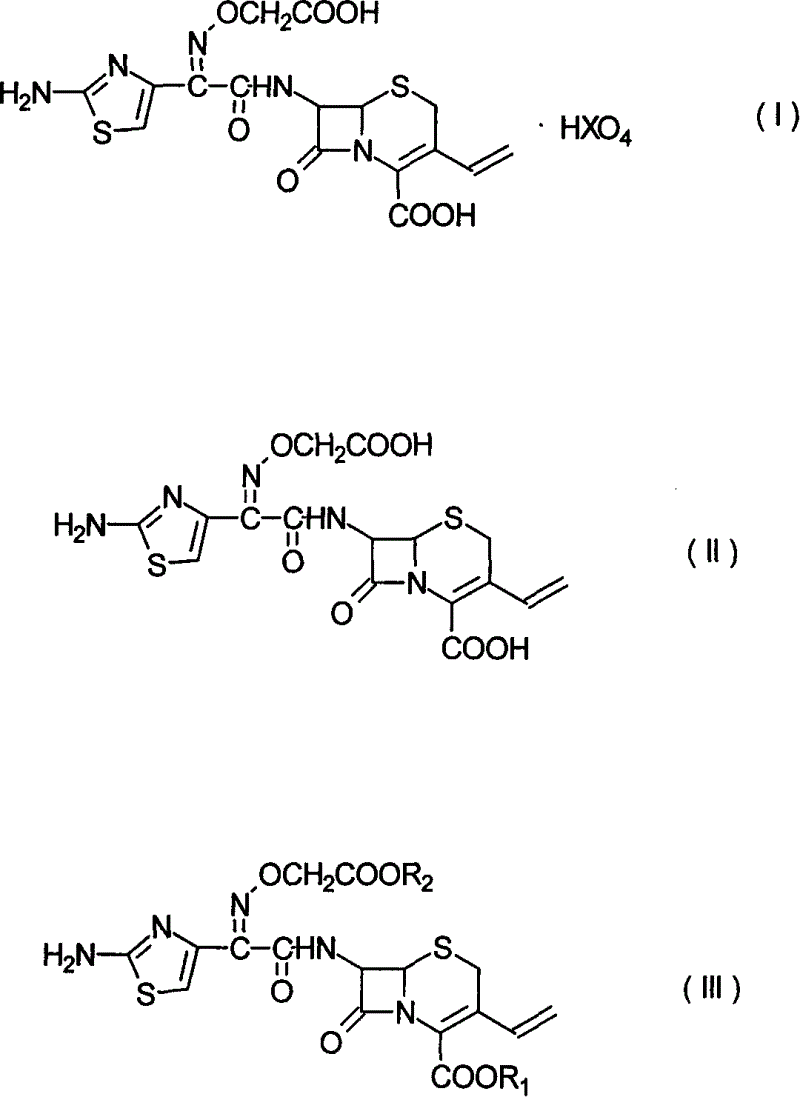
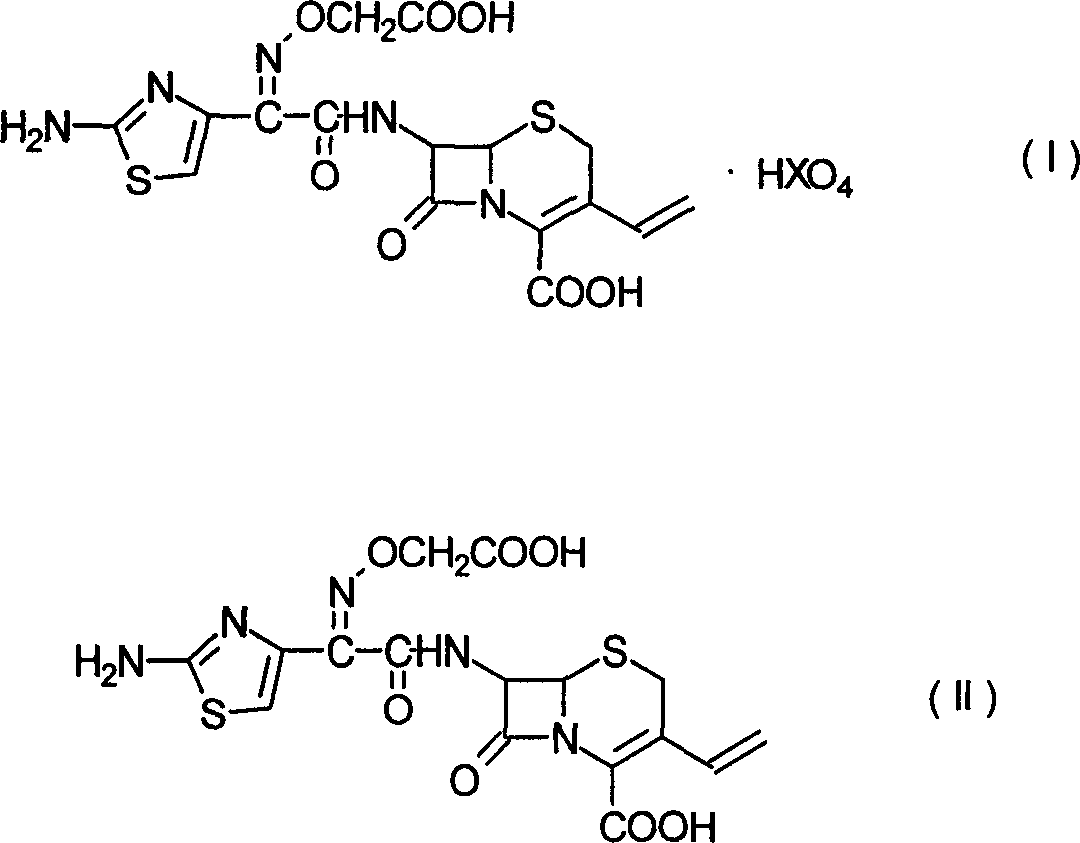
[0024] In a 500ml reaction flask, add 7-[[(2-amino-4-thiazole)-[(tert-butyloxyacetyl)oxime]acetyl]amino]-3-vinyl-8-oxo-5-thia -15g of 1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid [III], 150ml of dichloromethane, 21ml of formic acid, 12ml of perchloric acid, heated to 30°C and kept stirring for 2 hours. Cool down to below 5°C, precipitate a solid, filter, and dry to give light yellow 7-[[(2-amino-4-thiazole)-[(carboxymethyl)oxime]acetyl]amino]-3-vinyl-8-oxo - 5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid perchlorate [I] 16.5 g, yield 91.7%, HPLC 98%. 1 H-NMR (DMSO): 3.6, 3.8 (ABq, 2H), 4.69 (s, 2H), 5.20 (d, 1H), 5.31 (d, 1H), 5.59 (d, 1H), 5.79 (dd, 1H) , 6.92 (dd, 1H), 6.99 (s, 1H), 9.71 (d, 1H).
Embodiment 2
[0026] In a 500ml reaction flask, add 7-[[(2-amino-4-thiazole)-[(tert-butyloxyacetyl)oxime]acetyl]amino]-3-vinyl-8-oxo-5-thia -1-Azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid [III] 5g, ethyl acetate 100ml, formic acid 3ml, perchloric acid 2ml, heat up to 40°C and keep stirring for 1 hour. Cool down to below 5°C, precipitate a solid, filter, and dry to give light yellow 7-[[(2-amino-4-thiazole)-[(carboxymethyl)oxime]acetyl]amino]-3-vinyl-8-oxo -5.2 g of 5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid perchlorate [I], yield 87%, HPLC 98.2%. 1 H-NMR (DMSO): 3.6, 3.8 (ABq, 2H), 4.69 (s, 2H), 5.20 (d, 1H), 5.31 (d, 1H), 5.59 (d, 1H), 5.79 (dd, 1H) , 6.92 (dd, 1H), 6.99 (s, 1H), 9.71 (d, 1H).
Embodiment 3
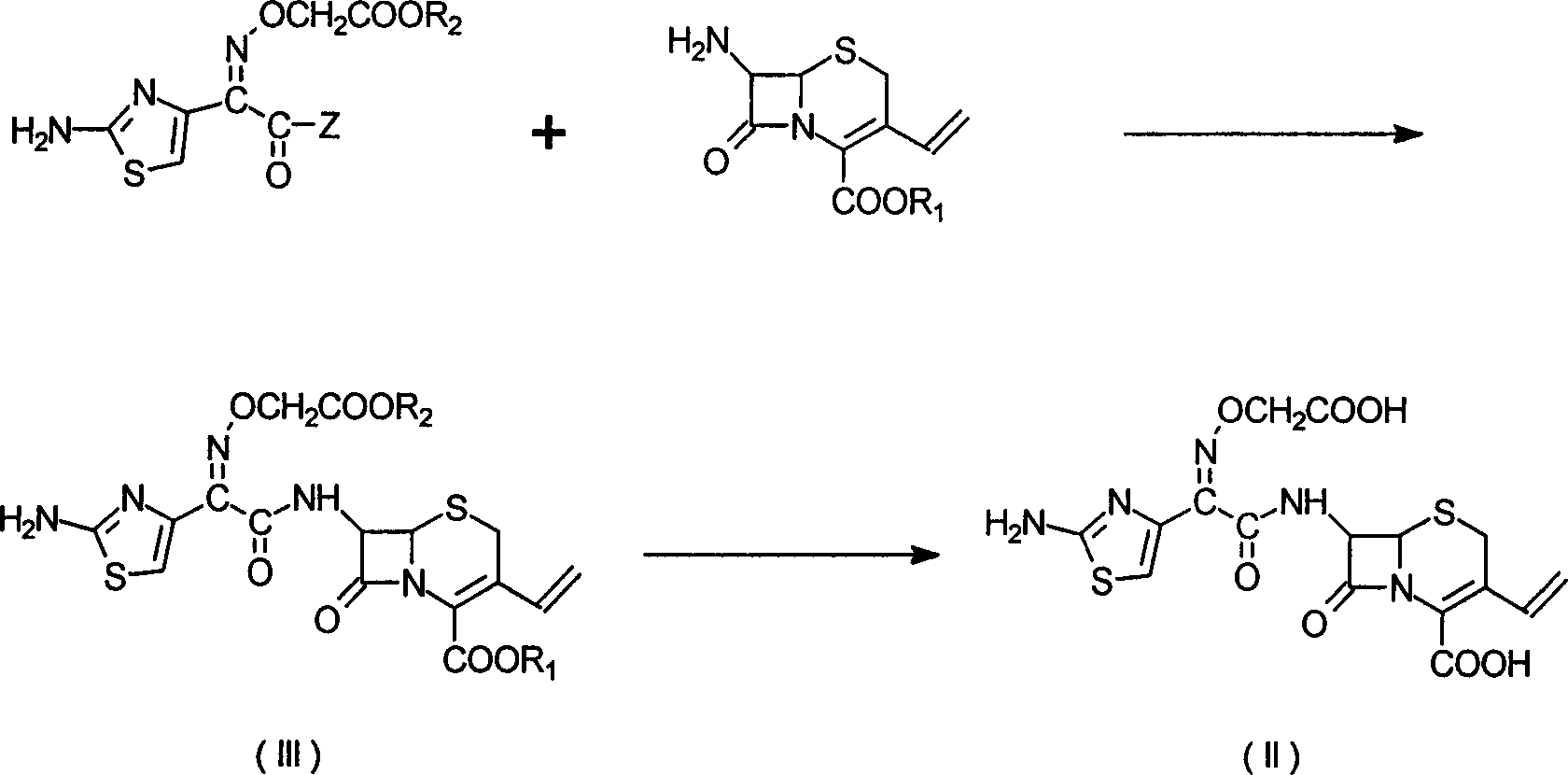
[0028] In a 500ml reaction flask, 7-[[(2-amino-4-thiazole)-[(carboxymethyl)oxime]acetyl]amino]-3-vinyl-8-oxo-5-thia-1 -Azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid perchlorate [I]16g was suspended in 150ml of water, the temperature was lowered to below 5°C, a saturated solution of sodium carbonate was added dropwise, and the solid was stirred and dissolved Afterwards, add carbon to decolorize for 1 hour. Filtrate, adjust the filtrate to pH 2.5 with 4N hydrochloric acid, stir at 5°C for 0.5 hours, filter out the solid, and dry to obtain 7-[[(2-amino-4-thiazole)-[(carboxymethyl)oxime]acetyl]amino 12 g of ]-3-vinyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (II, cefixime). HPLC 98.3%
[0029] 1 H-NMR (DMSO-D6): 3.54, 3.8 (ABq, 2H), 4.58 (s, 2H), 5.20 (d, 1H), 5.31 (d, 1H), 5.59 (d, 1H), 5.80 (dd, 1H), 6.80 (s, 2H), 6.86 (dd, 2H), 7.24 (s, 2H), 9.54 (d, 1H).
[0030] IR: 3536cm -1 , 3297cm -1 , 2948cm -1 , 3300-2500cm -1 , 1771cm -1 , 1668cm -1 , 1096cm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com