Methods for assessing patients with acute myeloid leukemia
An acute myeloid and leukemia technology, applied in the field of predicting the prognosis of patients with acute myeloid leukemia, can solve the problems of unseen response rate and differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Clinical evaluation and response definition
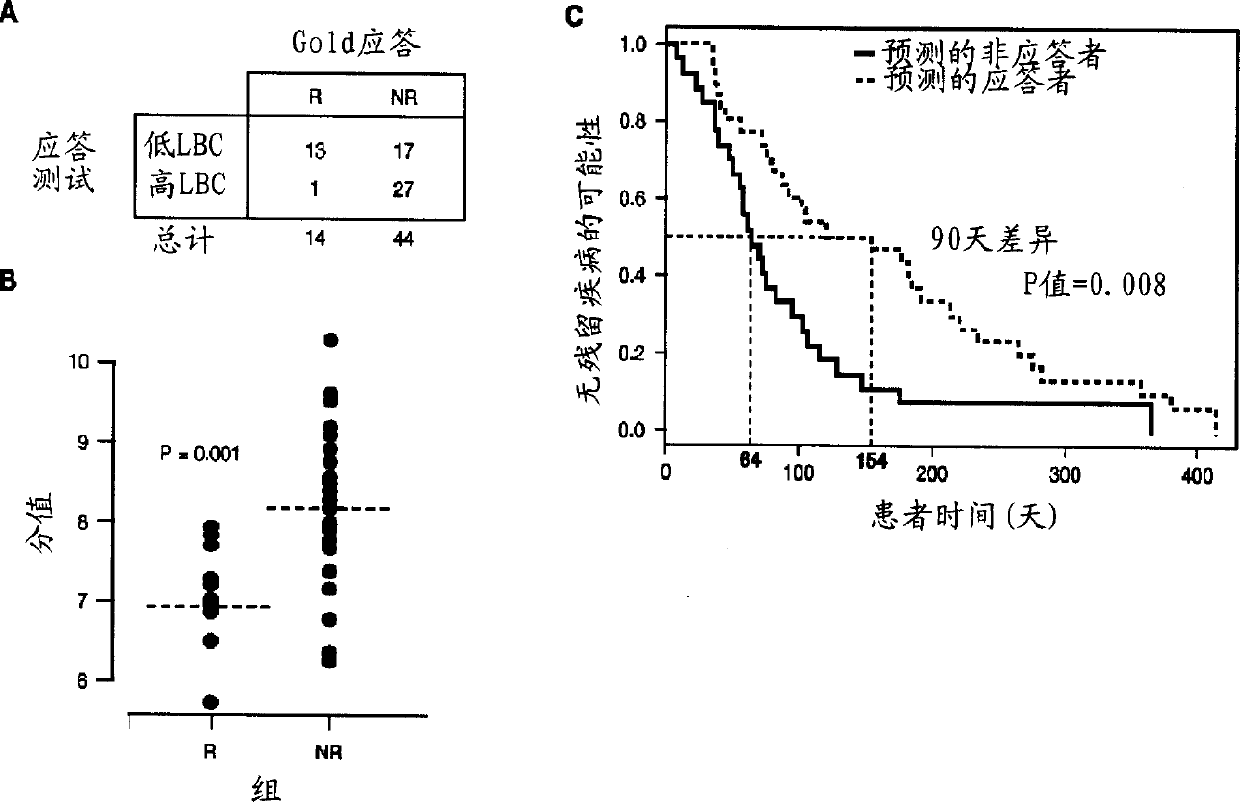
[0106] The current study is part of an open label, multi-center, non-comparative phase 2 clinical study in which tipifarnib was used at an initial oral dose of 600 mg twice a day (bid) Patients with relapsed or refractory AML (Harousseau et al. (2003)) are treated for 21 consecutive days at the beginning of each 28-day cycle. The patients were divided into 2 groups, namely those with relapsed AML and those with intractable AML. A total of 252 patients (135 relapsed and 117 refractory) were treated. Eight patients chose to provide bone marrow samples for RNA microarray analysis, for which individual consent was required. The overall response rate is relatively low in this study. Therefore, for the purpose of gene expression profiling, the response to tipifarnib is defined as an objective response (complete remission [CR], complete remission with incomplete platelet recovery [CRp] or partial remission [PR]). Patients with stable...
Embodiment 2
[0131] Identify genes that are differentially expressed between responders and non-responders
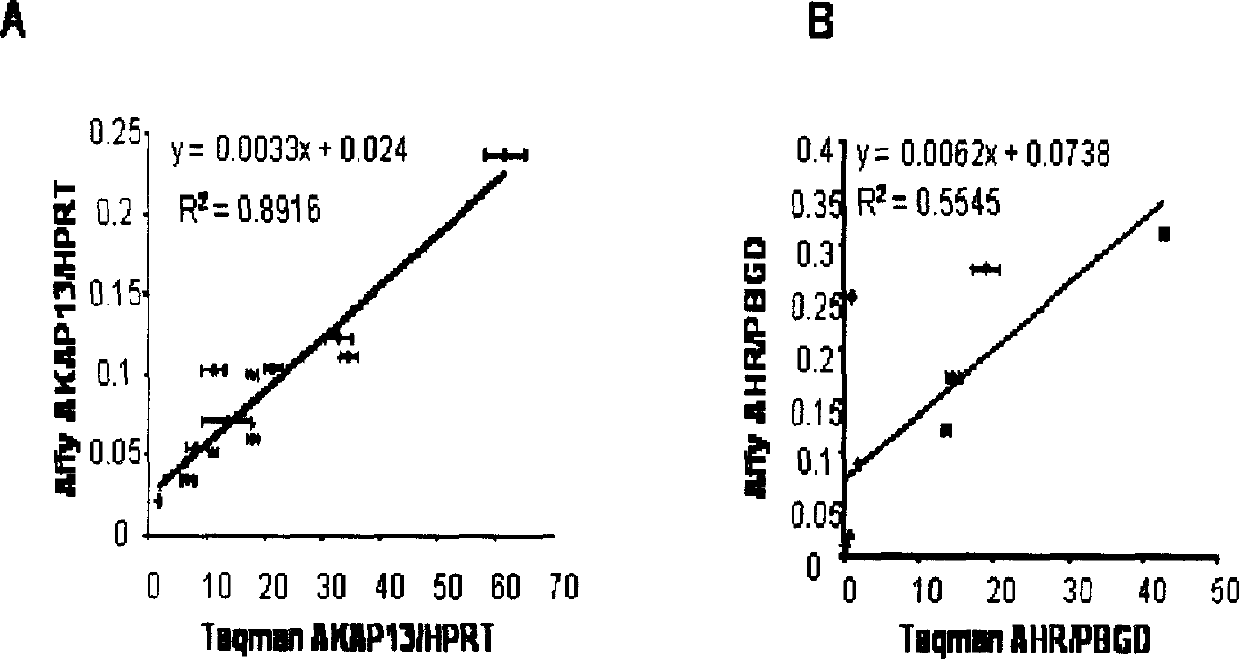
[0132] We next performed a supervised analysis using gene expression data to identify genes that are differentially expressed between all responders and at least 40% of non-responders. These criteria were selected to identify genes that can predict the response to tipifarnib with the highest level of sensitivity possible. From 11,723 genes, a total of 19 genes (Table 4) that were able to distinguish between responders and non-responders (Table 5) and gave a significant P value (P<0.05) in the t-test were identified. These genes include those involved in signal transduction, apoptosis, cell proliferation, tumorigenesis and, potentially, FTI biology (ARHH, AKAP13, IL3RA).
[0133] Seq ID NO
Gene symbol
Gene ID
Function description
5
1
6
7
8
9
10
11
12
13
14
15
AHR
AKAP13
MINA53
IDS
OPN3
GPR10...
Embodiment 3
[0152] Analysis of prognostic gene markers in AML
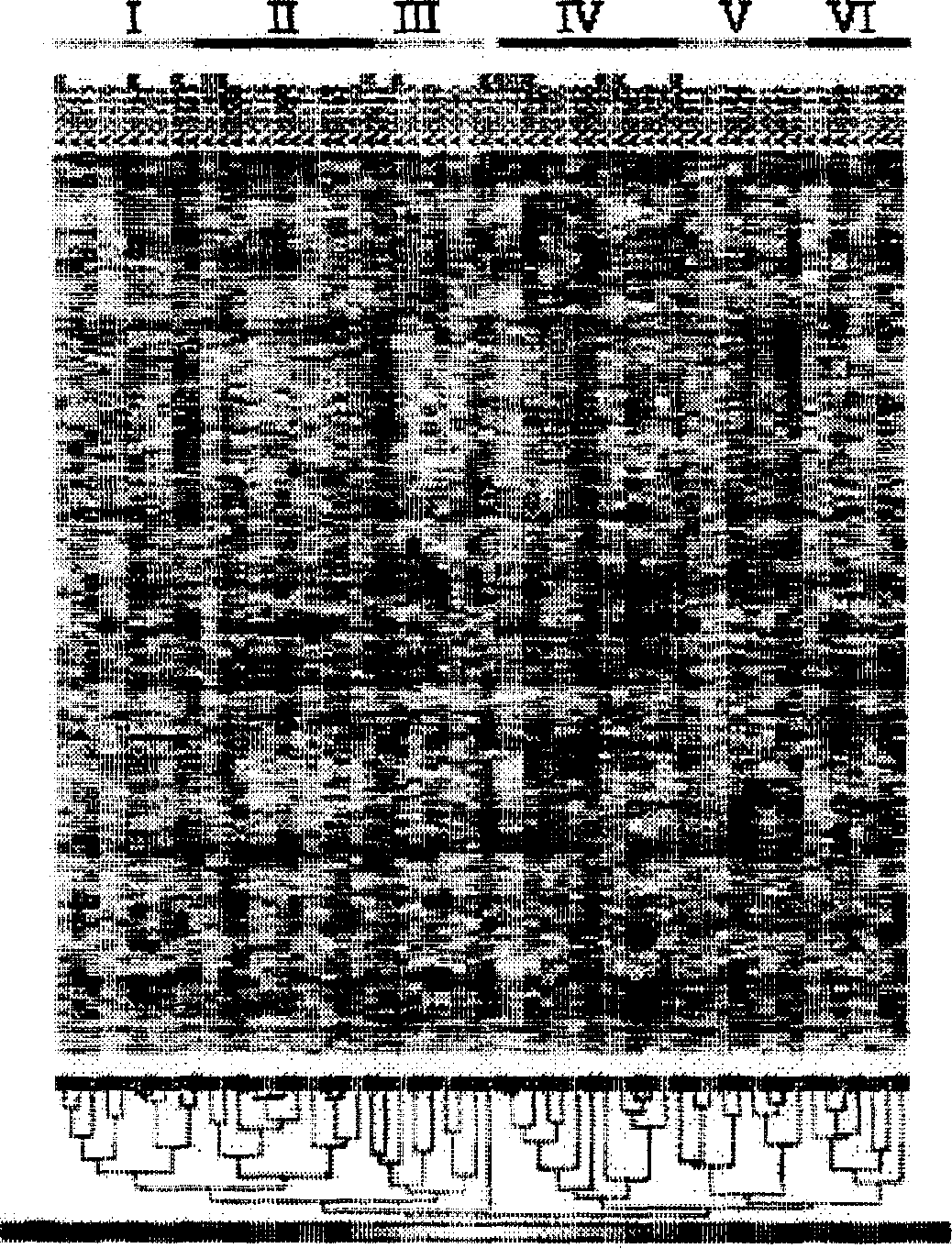
[0153] 3 Gene markers can predict prognosis that has nothing to do with the type of drug treatment. To measure this, we first evaluated gene expression markers recently identified in newly diagnosed AML patients who were treated with traditional chemotherapy. Bullinger et al. (2004). The cDNA array was used to define this marker, and we therefore first matched these genes with the probes present on the Affymetrix gene chip. The 167 probe sets of 133 predictive genes identified by Bullinger et al. (corresponding to 103 unique genes) were matched with the Affymetrix U133A chip. The 3 genes identified in our current analysis are not in the 133 gene list of Bullinger et al. SEQ ID NOs: 23-189. Two main patient groups were defined by using these 167 probe groups for hierarchical clustering (Figure 8A). Kaplan-Meier analysis showed that these clusters were clearly divided into patients with good and poor prognosis (Figure 8B, p=0.000...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com