Dosage forms comprising a cetp inhibitor and an HMG-CoA reductase inhibitor
A technology of reductase inhibitors and inhibitors, which can be used in medical preparations containing active ingredients, pill delivery, medical preparations with non-active ingredients, etc., and can solve problems such as loss of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
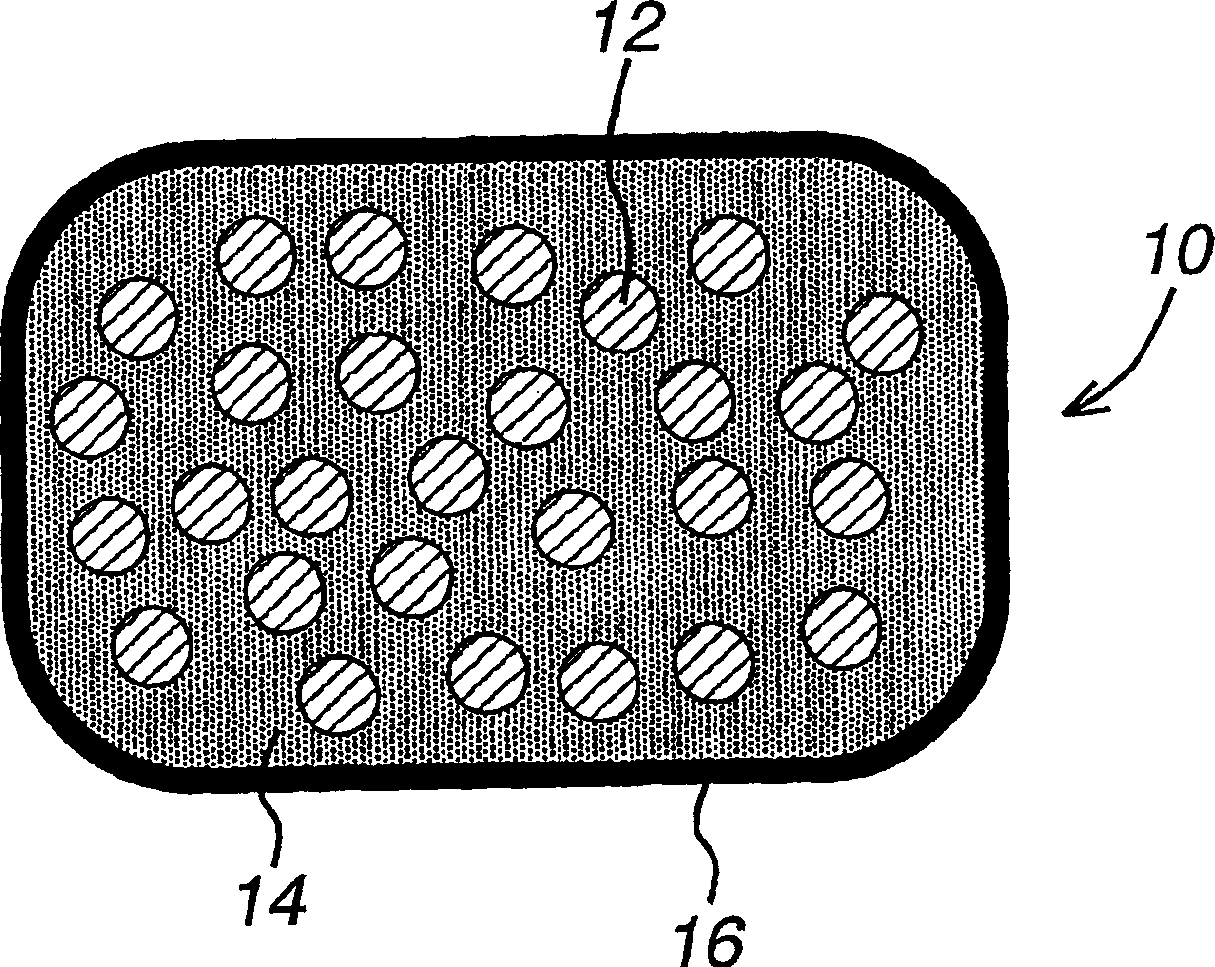
[1317] Granules of the HMG-CoA reductase inhibitor torvastatin and granules containing a solid amorphous dispersion of the CETP inhibitor and concentration-enhancing polymer were prepared separately. The two granules were combined and stored at 50°C and 75% relative humidity for 3 weeks. The stability of atorvastatin was measured and found to be increased relative to the control composition.
[1318] The following procedure was used to form a spray-dried product containing 25 wt% [2R,4S]-4-[(3,5-bis-trifluoromethyl-benzyl)-methoxycarbonyl-amino]-2-ethyl- 6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid ethyl ester (torcetrapib) and 75 wt% hydroxypropylmethylcellulose acetate succinate (medium size fraction, available from Shin Etsu located in Japan) (herein referred to as "HPMCAS-MG"). First, a spray solution containing 25 g torcetrapib, 75 g HPMCAS-MG and 900 g acetone was prepared. The spray solution was pumped with a high-pressure pump (ZenithZ-Drive 2000 Hig...
Embodiment 2 and 3
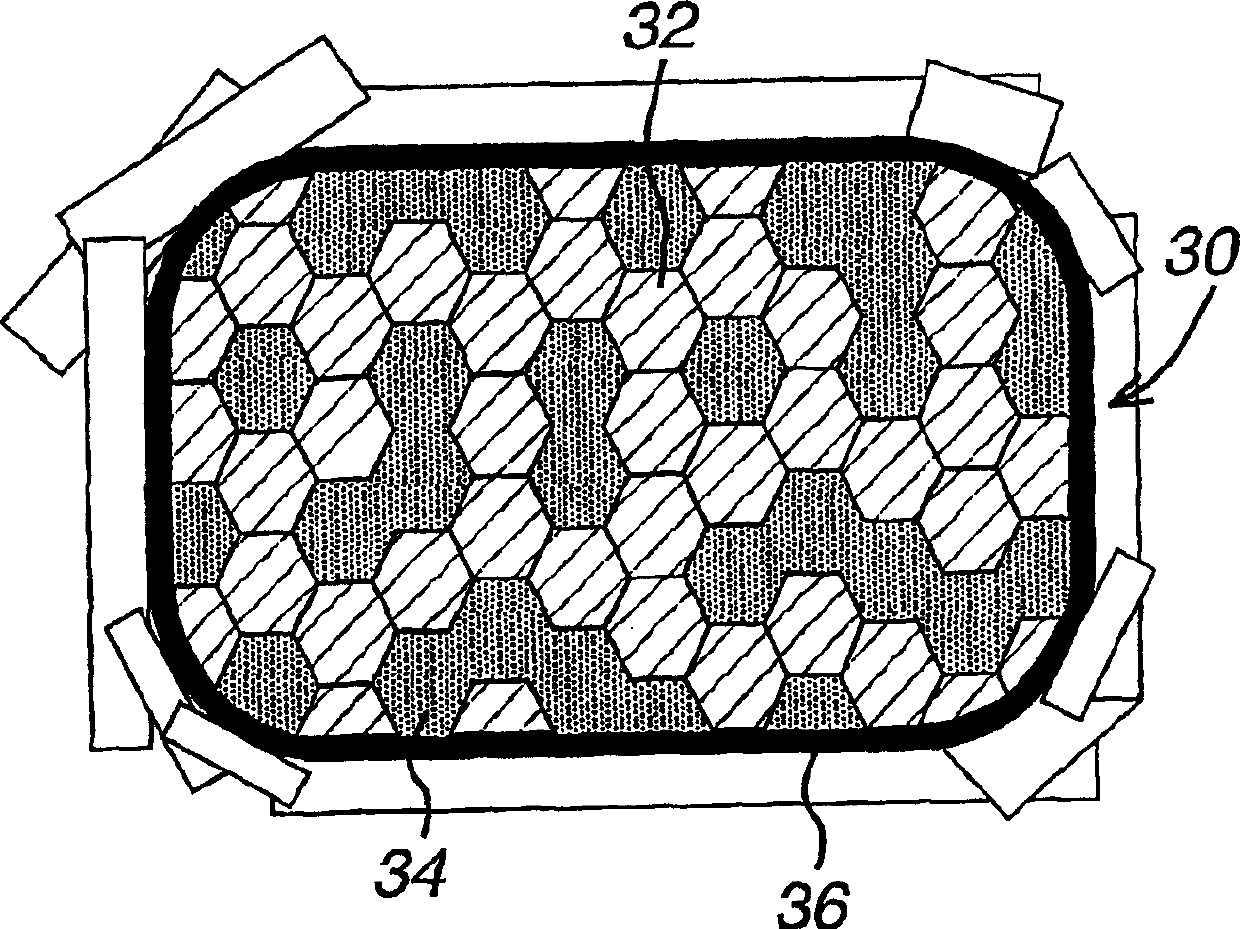
[1337] To make Example 2, equal weights of the granulated CETP inhibitor composition of Example 1 and the granulated HMG-CoA reductase inhibitor composition of Example 1 were blended as described in Example 1, from the blend Made into 200mg tablets. The acidic polymer HPMCAS accounts for 22.5wt% of Example 2, atorvastatin calcium accounts for 6.95wt% of Example 2, and the ratio of HPMCAS to atorvastatin is 3.24.
Embodiment 3
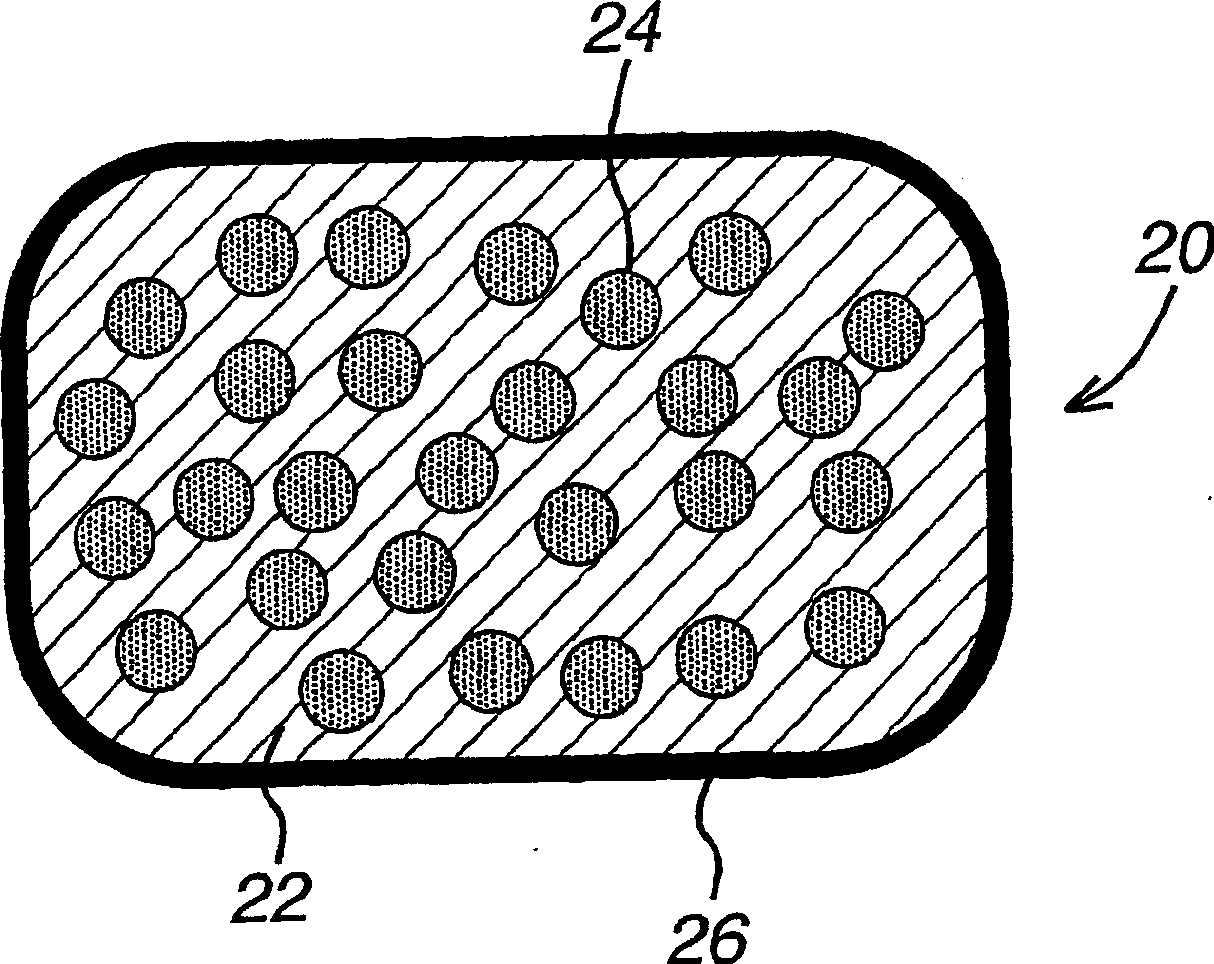
[1338] To make Example 3, tablets were made containing separate layers of the CETP inhibitor composition of Example 1 and the HMG-CoA reductase inhibitor composition of Example 1. Each tablet of Example 3 contained 400 mg of the dispersion granules in one layer and 288 mg of torvastatin granules in the second layer. The acid concentration-enhancing polymer HPMCAS accounted for 26.2wt% of Example 3, atorvastatin calcium accounted for 5.82wt% of Example 3, and the ratio of HPMCAS to atorvastatin was 4.5.
[1339] Examples 2 and 3 were stored at 50°C and 75% relative humidity for 3 weeks and analyzed by HPLC as described above. The results are shown in Table 5.
[1340] sample
Degradation degree (wt%)
Example 2
0.09
Example 3
0.04
[1341] Example 2 shows that tablets prepared by first forming separate granules (one containing a solid amorphous dispersion and one containing torvastatin) and then blending the granules into tablet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com