Receptor function controlling agent
A technology of regulators and functions, applied in the fields of organic chemistry, digestive system, metabolic diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
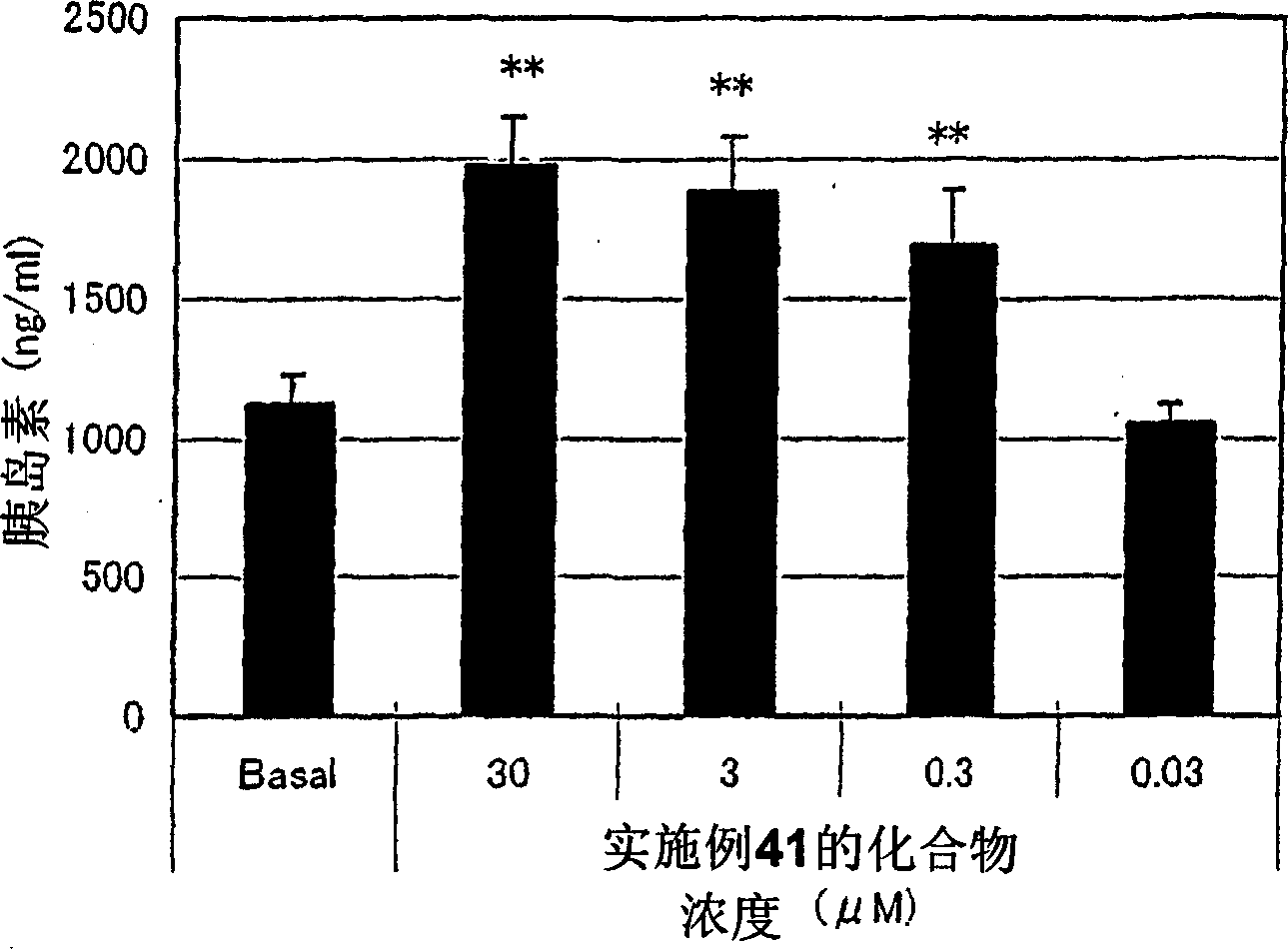
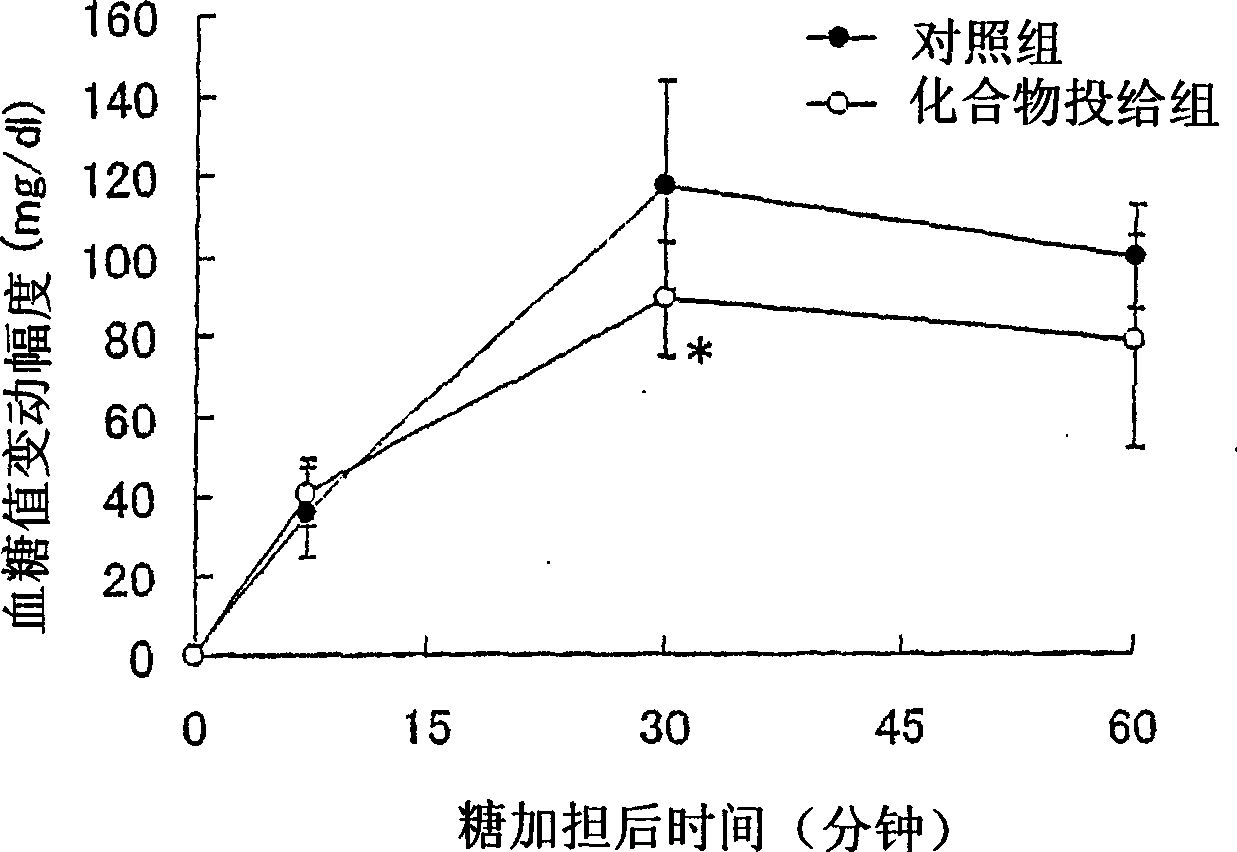
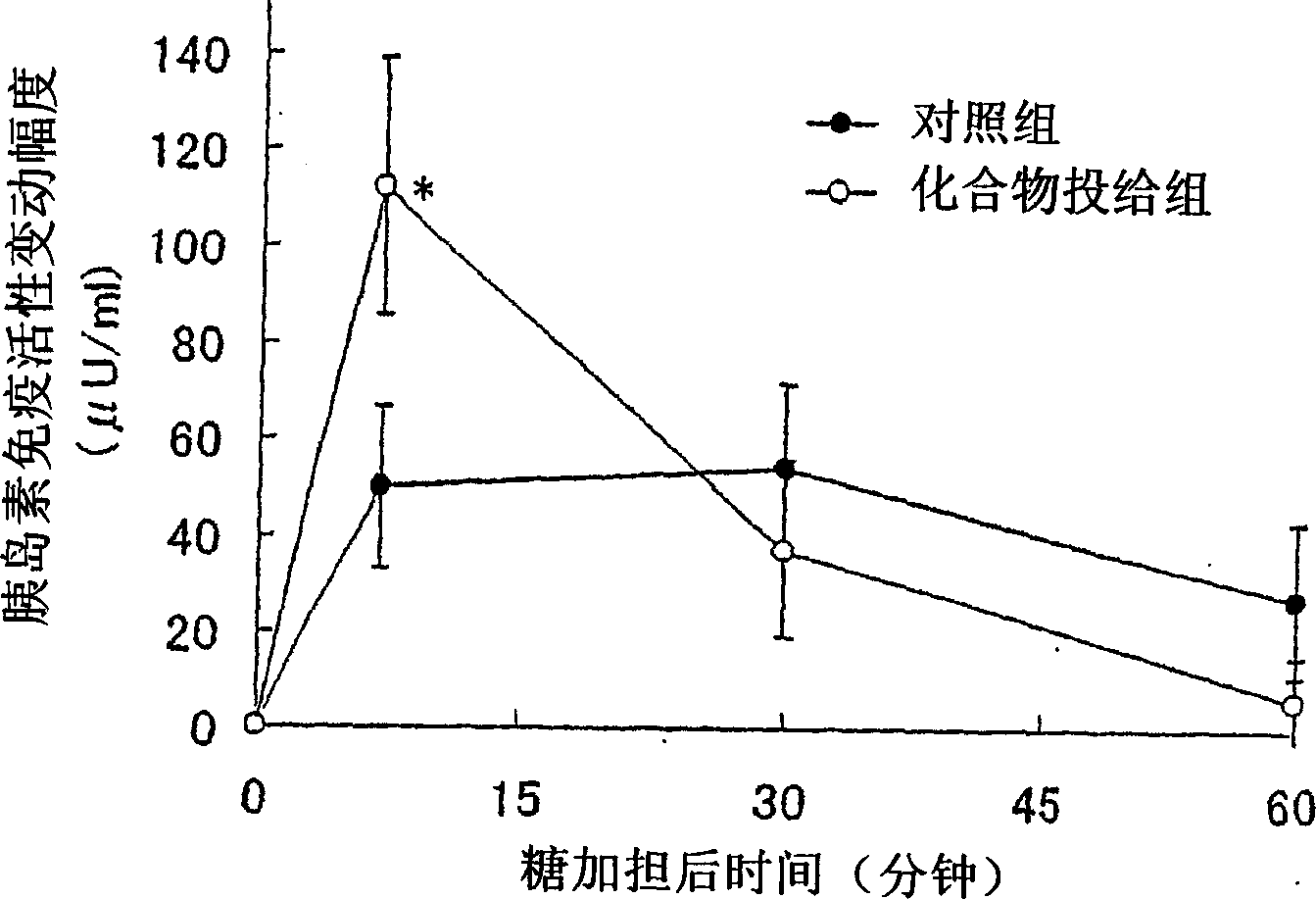
Embodiment 312
[0542] 3-(4-((4-(((2-phenoxypropyl)amino)methyl)benzyl)oxy)phenyl)propanoic acid (Example 312);
Embodiment 330
[0543] 3-(4-((4-((Dibenzylamino)methyl)benzyl)oxy)phenyl)propanoic acid (Example 330);
Embodiment 334
[0544] 3-(4-((4-(((2-imidazo[1,5-a]pyridin-3-ylethyl(phenyl)amino)methyl)benzyl)oxy)phenyl)propanoic acid (Example 334);
[0545] 3-(2-(4-((3-phenoxybenzyl)oxy)phenyl)ethyl)-1,2,4-oxadiazol-5(4H)-one (Reference Example 213).
[0546] As compounds that can be used in the present invention, JP-A Nos. 2002-265457, 2002-212171, 2001-226350, 2001-199971, 2000-198772, Compounds described in JP-A-2000-80086, JP-A-2000-34266, JP-A-09-323983, JP-A-08-311065, etc.
[0547] Examples of salts of compounds that can be used in the present invention include metal salts, ammonium salts, salts with organic bases, salts with inorganic acids, salts with organic acids, salts with basic or acidic amino acids, etc. . Preferable examples of metal salts include, for example, alkali metal salts such as sodium salts and potassium salts; alkaline earth metal salts such as calcium salts, magnesium salts, and barium salts; and ammonium salts. Preferable examples of salts with organic bases include tri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com