Expression vectors comprising the MCMV IE2 promoter
A technology for expressing vectors and promoters, applied in vectors, using vectors to introduce foreign genetic material, viruses/bacteriophages, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0140] Example 1: Evaluation of expression vectors in transient transfection
[0141] Materials and methods:
[0142] Material
[0143] Cells: CHO-S from Gibco / Invitrogen (Cat no 11619).
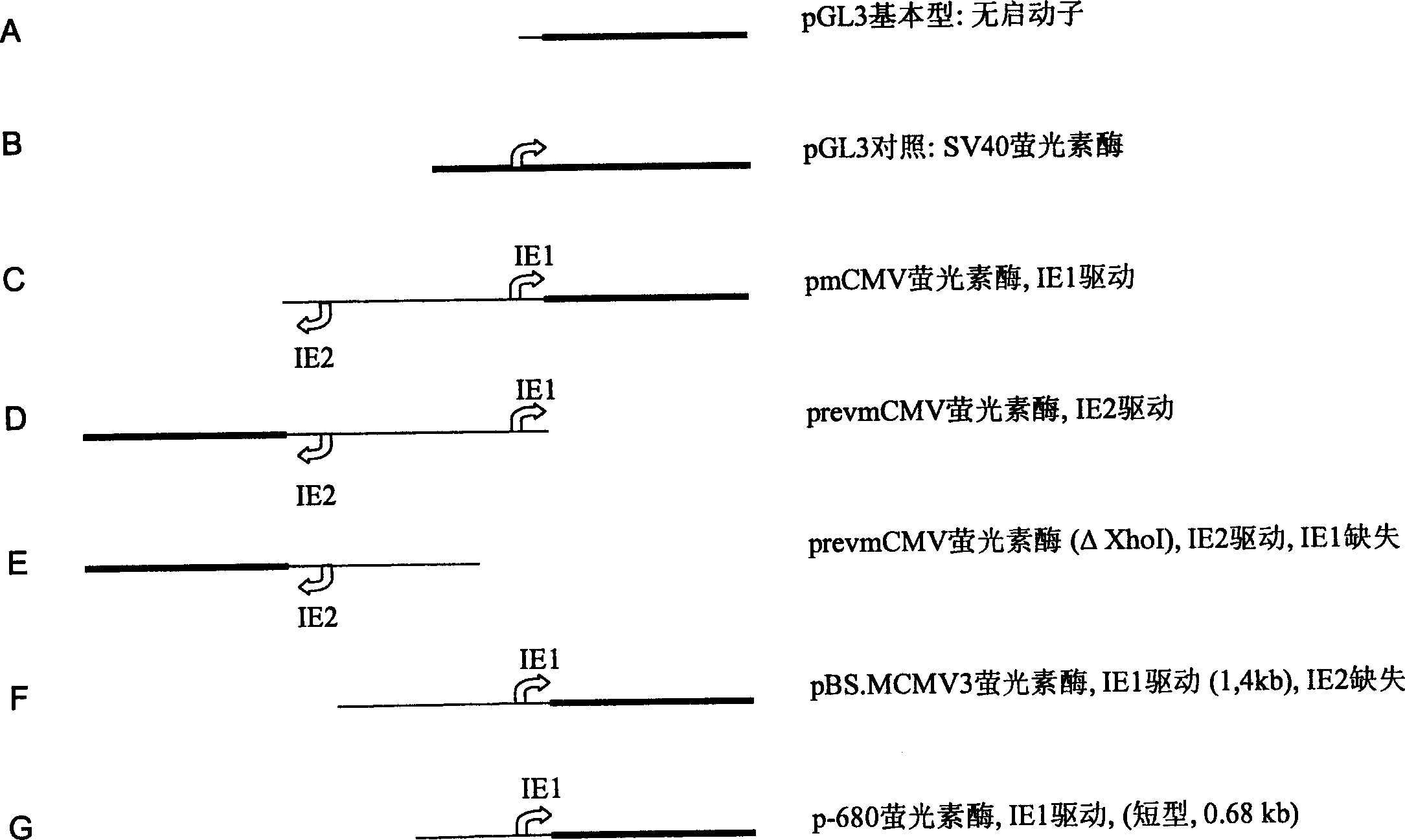
[0144] Following the manufacturer's protocol, isolate such as figure 2 and image 3 Plasmid DNA as indicated.
[0145] Transfection:
[0146] Transfection reagent: Lipofectamine (Invitrogen, Cat No 18324-012)
[0147] Format: 24-well plate
[0148] Cells: CHO-S cells in exponential growth phase were passaged 24 hours before transfection. To avoid a stationary phase of low cell density, dilute the cells to 0.75 x 10 6 cells / ml. The total amount of cells to be transfected is 1.5 x 10 5 , resuspended in a 24-well plate with 100 μl of serum-free medium SFM II (Invitrogen, Cat No 12052-114) per well.
[0149] The transfection mix was as follows:
[0150] A) Lipofectamine: 2μl
[0151] SFM II medium: 48 μl
[0152] Total volume: 50μl
[0153]B) DNA: 1 μg (50ng expression vector...
Embodiment 2
[0165] Example 2: Evaluation of expression vectors in stable transfection
[0166] Materials and methods:
[0167] method:
[0168] Cells: CHO-S from Gibco / Invitrogen (Cat no 11619).
[0169] Plasmid DNA ( figure 2 ).
[0170] Transfection:
[0171] Transfection reagent: Lipofectamine (Invitrogen, Cat No 18324-012)
[0172] Stable transfection uses T75 flasks. CHO-S cells in exponential growth phase were passaged 24 hours before transfection. To avoid a stationary phase of low cell density, dilute the cells to 0.75 x 10 6 cells / ml. The total amount of cells to be transfected is 5 x 10 6 , resuspended in 7 ml of serum-free medium SFM II (Invitrogen, Cat No 12052-114) in a T75 flask.
[0173] The transfection mix was as follows:
[0174] A) Lipofectamine: 52.1μl
[0175] SFM II medium: 517.9 μl
[0176] Total volume: 570 μl.
[0177] B) DNA: 10 μg linearized plasmid DNA, (9 μg luciferase expression vector + 1 μg selection plasmid: SV40 promoter driving the pur...
Embodiment 3
[0189] Example 3: Co-expression of two polypeptides of interest with bidirectional expression vectors
[0190] Materials and methods:
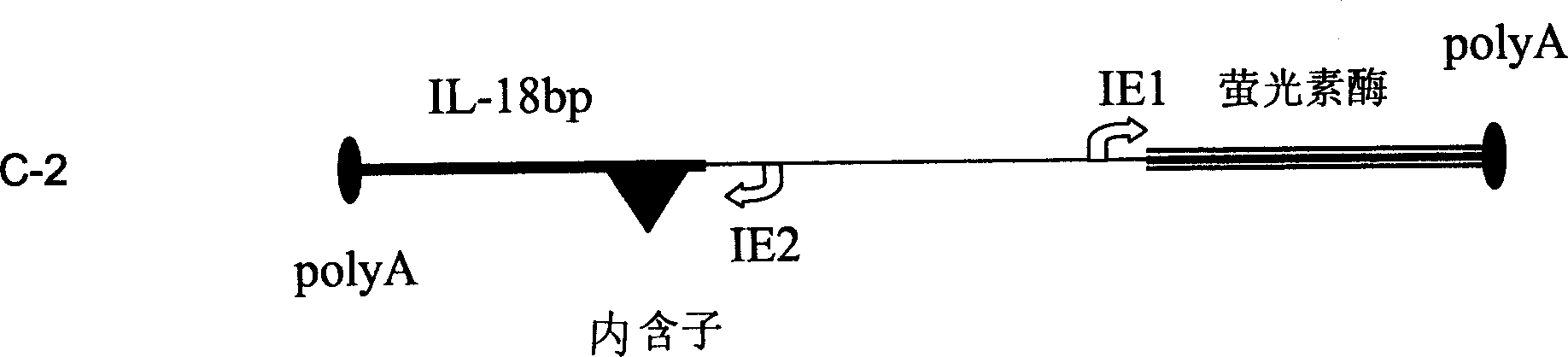
[0191] Transfection was carried out with reference to the method of Examples 1 and 2. Briefly, 900, 500, 300 and 100 ng of vector DNA (construct C-2, see image 3 ) transiently transfected CHO-S cells (suspension, Gibco SFMII) (each condition was repeated three times). Two days after transfection, cell extracts were assayed for luciferase in RLU (relative luminance units) in triplicate. The supernatants from these wells were removed before the cells were lysed, confluent, and analyzed for IL-18BP by ELISA (see below).
[0192] ELISA test of IL-18BP
[0193] The amount of recombinant human IL-18BP (rhIL-18BP) in the supernatant was determined in a standard ELISA assay using a biotin-conjugated and protein G-purified monoclonal anti-rh-IL-18BP antibody. Extravidine-HRP (Sigma) was used as the detection reagent.
[0194] Figure 9 Shown is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com