Anti-cancer anthracycline drug-antibody conjugates
An anthracycline and conjugate technology, applied in the field of therapeutic conjugates, can solve problems such as damage, toxicity, large non-target tissue, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Embodiment 1: the synthesis of 2-PDOX
[0094] Synthesis of 2-pyrrolinyl-doxorubicin (2-PDOX): 4-iodobutyraldehyde: 2-(3-chloropropyl)-1,3-di-oxolane (1.3 mL; 10 mM) dissolved in 200 mL of acetone containing 30 g of sodium iodide (200 mmol; 20-fold excess). The solution was refluxed for 24 hours and evaporated to dryness. The crude mixture was used in the following reactions. Doxorubicin hydrochloride (550 mg, 946 μmol) was dissolved in 6.5 ml of DMF, and 3.86 g (19.48 mmol, 20-fold excess) of 4-iodobutyraldehyde was added, followed by 500 μl of N , N-diisopropylethylamine (DIPEA). After five minutes, the material was purified by reverse phase HPLC on a Waters NovaPak C-18 column with gradient elution. The gradient was: 90:10 eluent A to 70:30 eluent B, 75 ml per minute, over 40 minutes, where eluent A was 0.1% trifluoroacetic acid (TFA) and eluent B contained 0.1 % TFA in 90% acetonitrile. Electron spray mass spectrometry confirmed the identity of the product, M+...
Embodiment 2
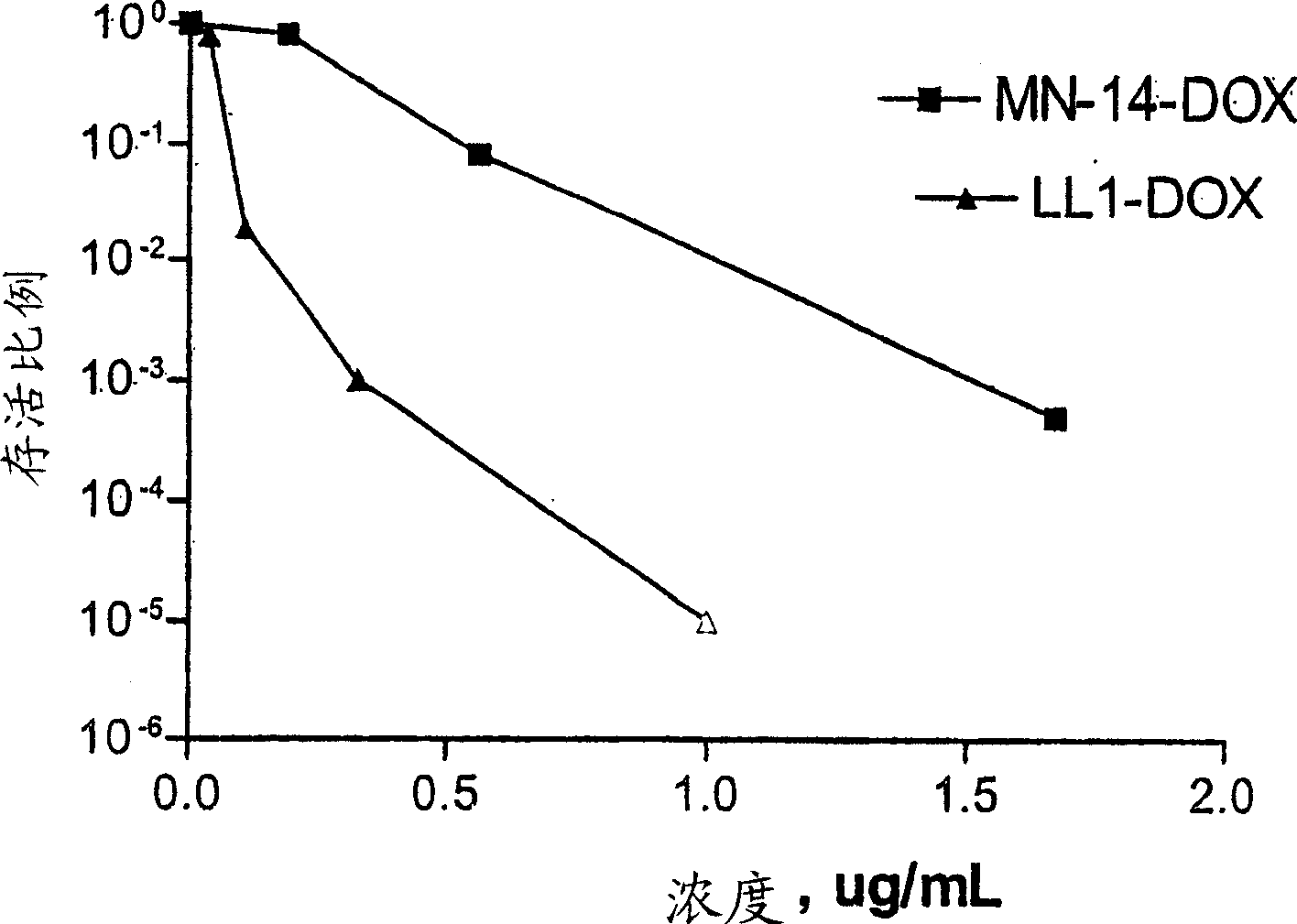
[0095] Example 2: Conjugation of 2-PDOX to anti-CD22 antibody humanized LL2 (hLL2)
[0096] a) Activation of 2-PDOX: 2-PDOX (5.95 mg; 1×10 -5 mol) with one molar equivalent of the commercially available linker 4-(N-maleimidomethyl)cyclohexane-1-carboxyhydrazide (M 2 C 2 H; Pierce Chemical Co., Rockford, IL) (2.88 mg; 1 x 10 -5 mol) in 0.5 mL of dimethyl sulfoxide (DMSO). The reaction mixture was heated at 50-60°C for 30 minutes under reduced pressure. The desired product was purified by preparative RP-HPLC using a gradient consisting of 0.3% ammonium acetate and 90% acetonitrile, 0.3% ammonium acetate in pH 4.4, eluting from mostly unreacted 2-PDOX (early about 0.5 min) and separation of the desired product from unreacted M2C2H (eluting earlier). The amount recovered was estimated by reference to the UV absorbance level of the sample (496 nm) relative to a standard solution of 2-PDOX in acetonitrile / ammonium acetate buffer. If not used immediately, maleimide-activated 2-...
Embodiment 3
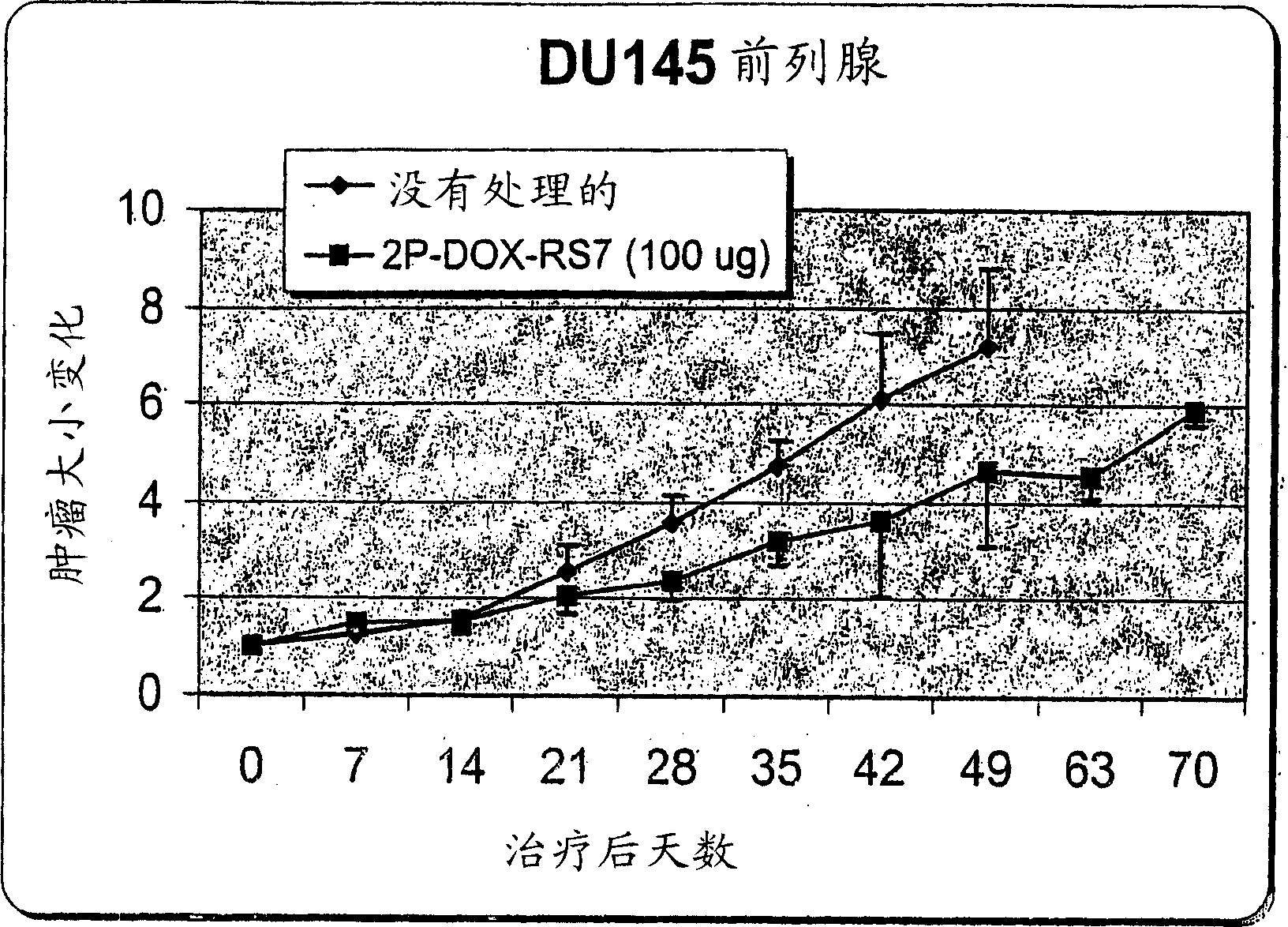
[0099] Example 3: Conjugation of 2-PDOX to anti-CD74 antibody humanized LL1 (hLL1)
[0100] a) Activation of 2-PDOX: 2-PDOX (5.95 mg; 1×10 -5 mol) with one molar equivalent of the commercially available linker 4-(N-maleimidomethyl)cyclohexane-1-carboxyhydrazide (M2C2H; Pierce Chemical Co., Rockford, IL) ( 2.88 mg; 1 x 10 -5 mol) in 0.5 mL DMSO. The reaction mixture was heated at 50-60°C for 30 minutes under reduced pressure. The desired product was purified by preparative RP-HPLC using a gradient consisting of 0.3% ammonium acetate and 90% acetonitrile, 0.3% ammonium acetate in pH 4.4, eluting from mostly unreacted 2-PDOX (early about 0.5 min) and separation of the desired product from unreacted M2C2H (eluting earlier). The amount recovered was estimated by reference to the UV absorbance level of the sample (496 nm) relative to a standard solution of 2-PDOX in acetonitrile / ammonium acetate buffer. If not used immediately, maleimide-activated 2-PDOX was frozen and lyophili...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com