Use of compounds for the prevention of drug-induced cell toxicity
A cytotoxicity, compound technology, applied in the directions of heterocyclic compound active ingredients, drug combinations, antitoxic agents, etc., can solve the problems of reducing cytotoxicity, not disclosed, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
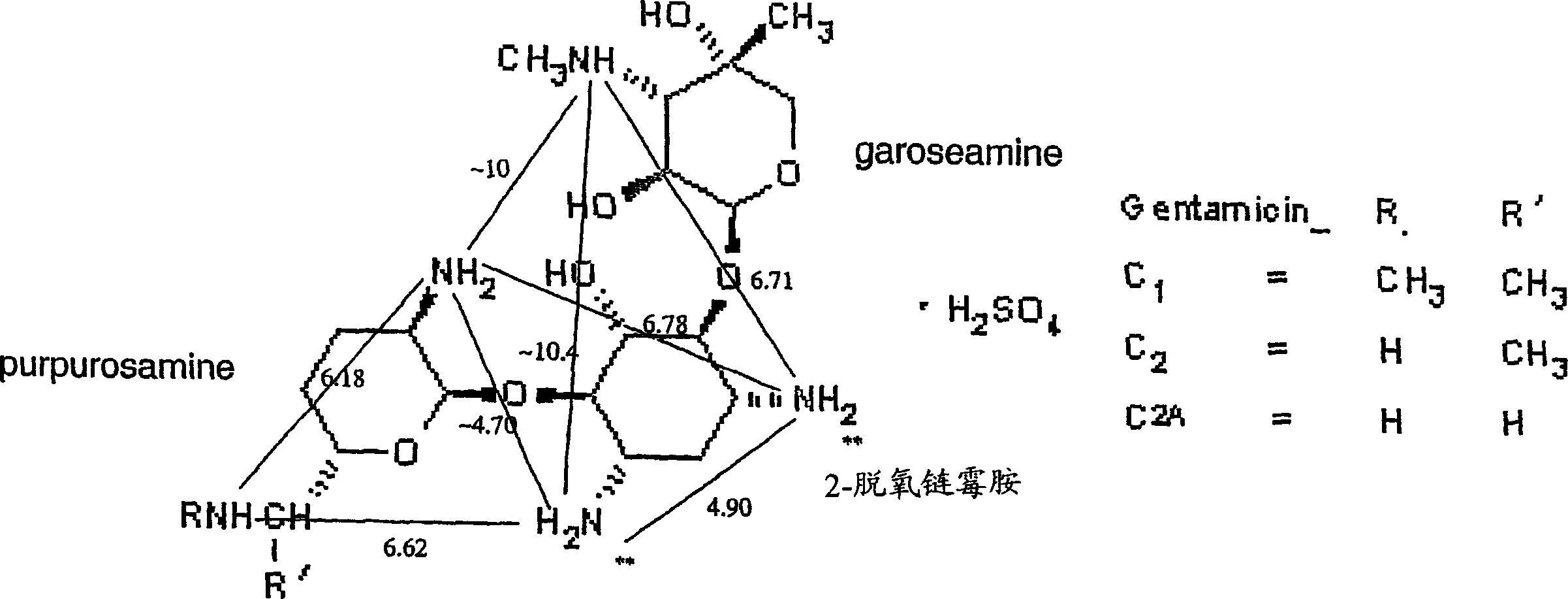
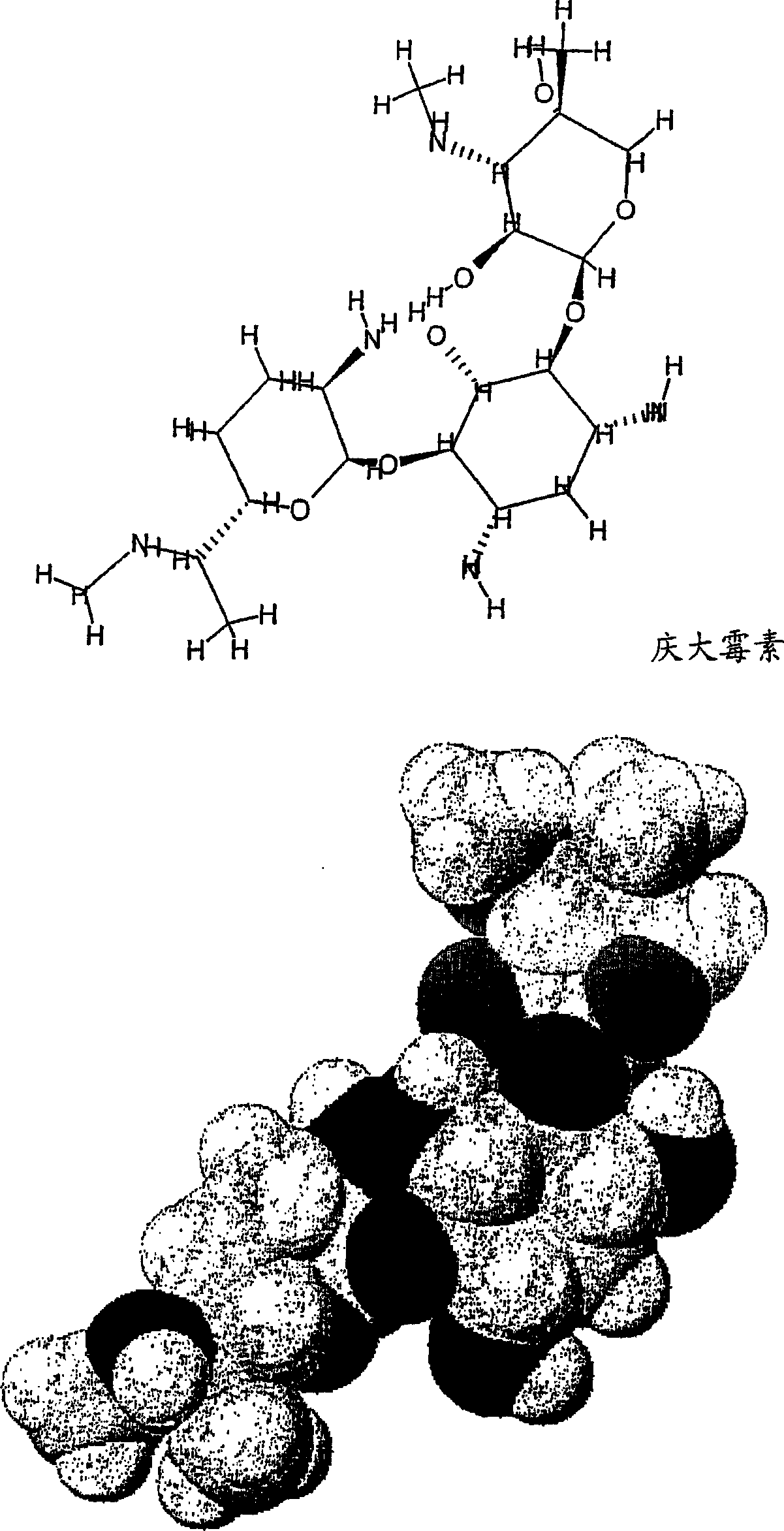
[0232] The effect of the compound of the invention 2-[4-(2-aminoethyl)piperazin-1-yl]ethylamine (Aldrich, D2, 340-8, lot 06028BO-332) was tested in vivo. The uptake of gentamicin in the kidneys of mice following intraperitoneal (i.p.) administration of the compound was determined.
[0233] For the normal administration of radioactive gentamicin, the following protocol was followed: 50 mg / kg tritiated gentamicin and 3 mg inhibitor or no 3 mg inhibitor were injected intraperitoneally per mouse. This is equivalent to the clinical dose of gentamicin used in 1% of patients.
[0234] To compete for the clinical dose of gentamicin, each mouse was injected with a total of 3 mg of 2-[4-(2-aminoethyl)piperazin-1-yl]ethylamine combined with 50 mg / kg tritiated gentamicin A mixture of gentamycin and 5 mg / kg non-radioactive gentamicin (clinical dose). The control group was given gentamicin only. Compared with the control group, the inhibition rate was 40%.
Embodiment 2
[0236] in vitro test
[0237] Interactions between gentamicin and inhibitors were assessed by proton surface resonance (SPR) analysis on a Biacore 2000 instrument. Megalin receptors were purified from rabbit kidneys and fixed at 28-40 fmol / mm as described by Bim et al. 2 concentration. Sample dissolved in 10mM Hepes, 150mM NaCl, 1.5mM CaCl 2 , 1mMEGTA, 0.005% Tween-20pH 7.4. The same buffer was used as running buffer. After each analysis, regeneration of the sensor chip was performed with 1.6M glycine-HCl buffer pH 3.0. Biacore responses are expressed in relative response units (RU), the difference in response between the protein and the control fluidic channel. Samples contained 2 mM gentamicin and 0-10 mM or 0-20 mM inhibitor. Responses were recorded at the maximum and corrected for the effect of inhibitor responses.
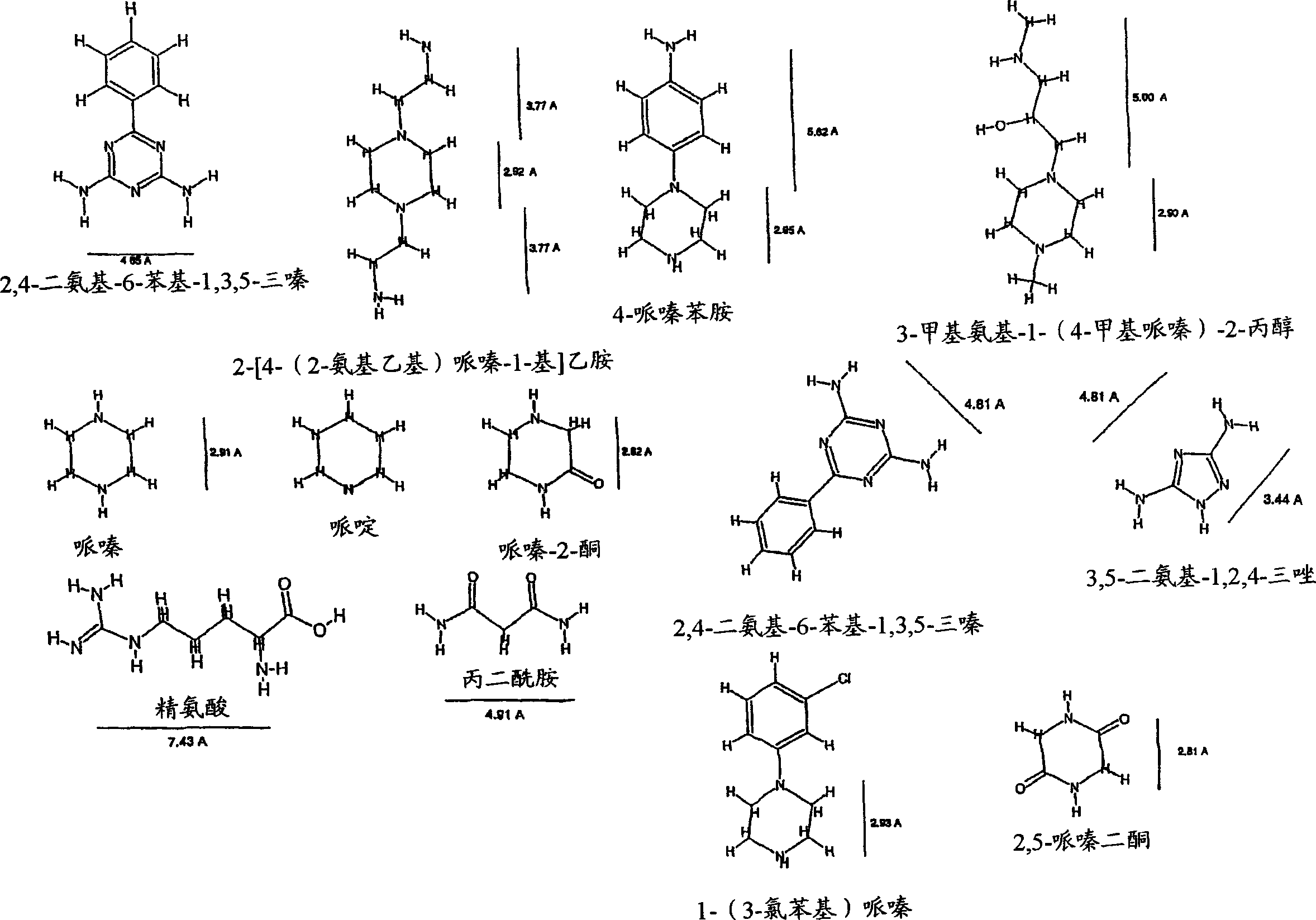
[0238] The test detected 3,5-diamino-1,2,4-triazole, malonamide, piperidine, 2,5-piperazinedione, piperazine, 3-methylamino-1-(4-methyl Inhibitory eff...
Embodiment 3
[0240] By the evaluation method described in Example 2, this test shows the inhibitory effect of diaminoalkanes. The results are shown in Figure 4 middle.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com