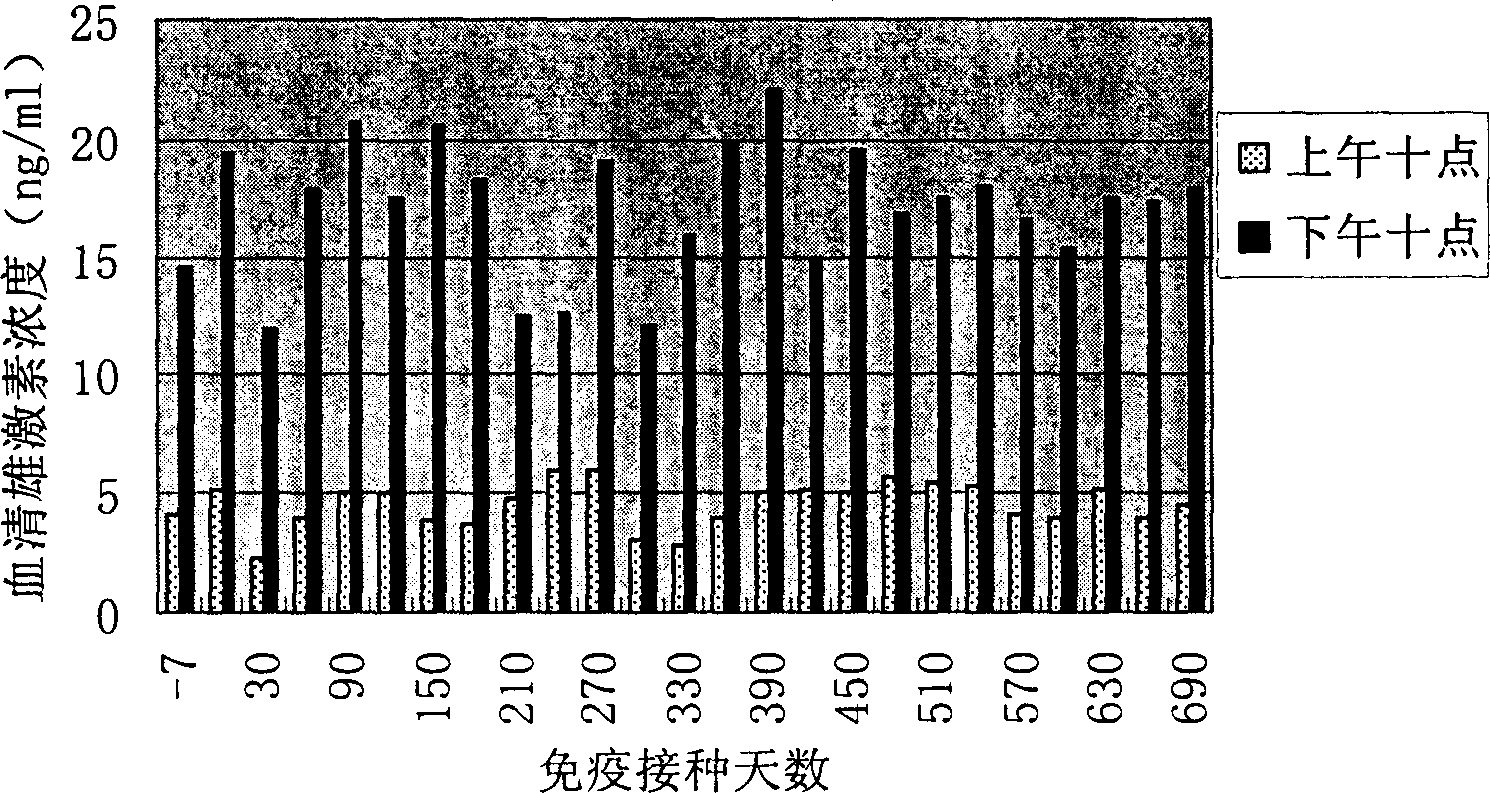
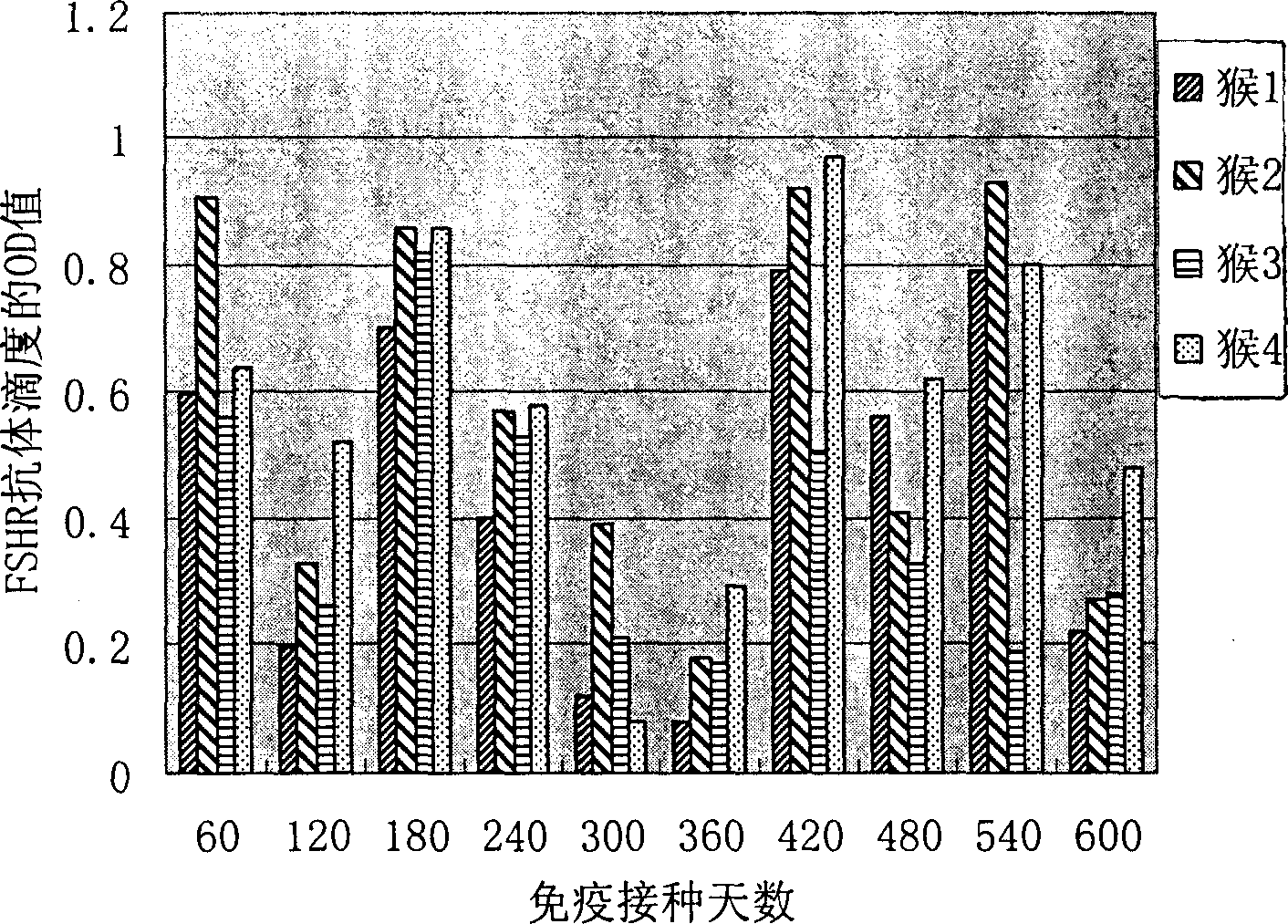
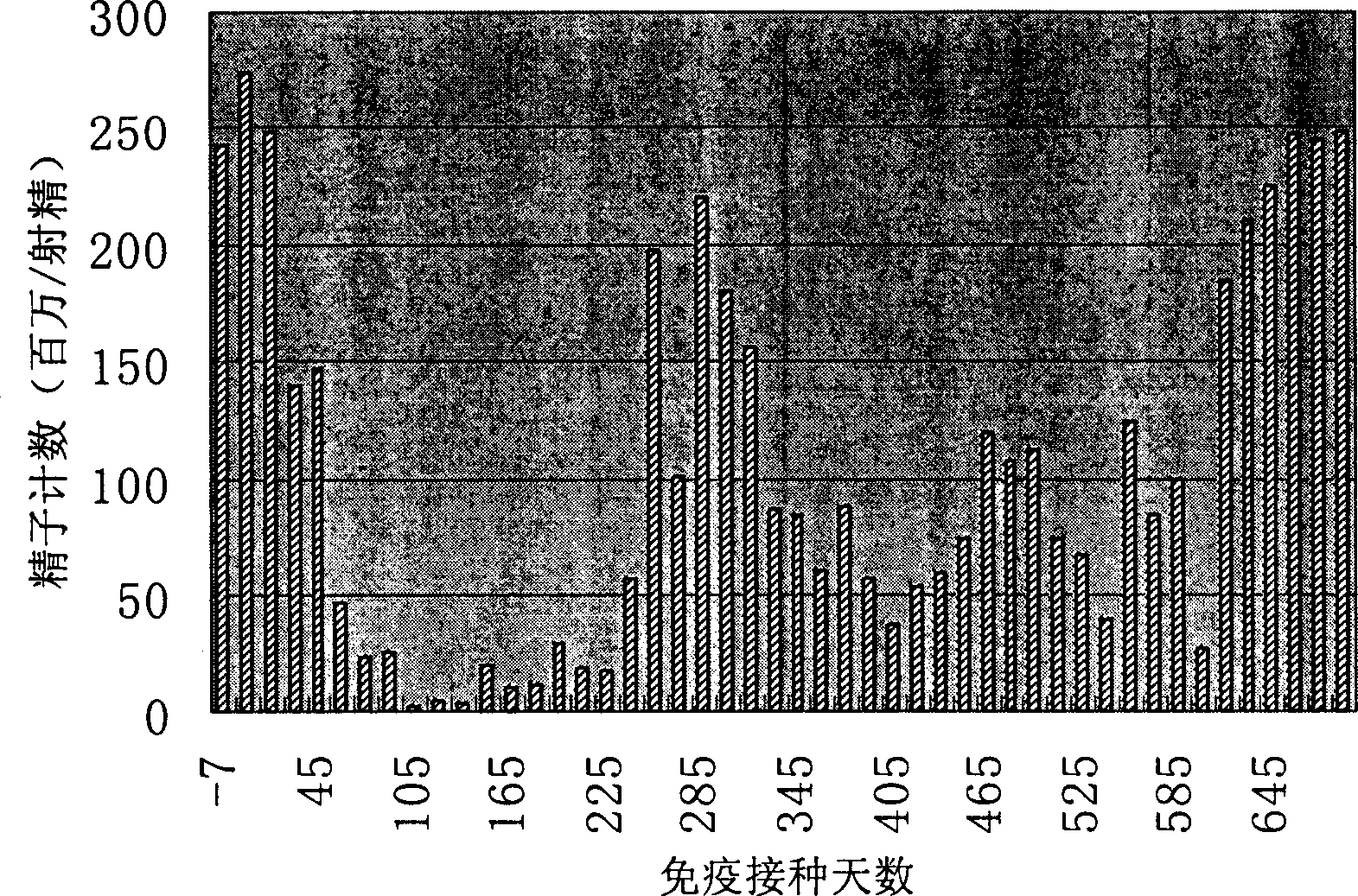
Follicle stimulating hormone receptor(FSHR) specific binding region polypeptide, its expression vector, preparation method and application in drug preparation
A follicle-stimulating hormone and receptor-specific technology, applied in the field of genetic engineering, can solve problems such as the advent of male contraceptives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] 1. Construction of phage expressing FSH receptor polypeptide.
[0065] Get the double-stranded oligonucleotides (respectively corresponding to SEQ ID No.1, SEQ ID No.3 and SEQ ID No.1, SEQ ID No.3 and SEQ ID No.6) of coding human FSHR three groups of polypeptides (SEQ ID No. ID No.5), these three pairs of oligonucleotides were linked into the enzyme cutting sites of PstI and HindIII restriction endonucleases respectively. Treat the f88-4 phage with the above two restriction endonucleases, 37°C for 30 minutes, add the above three pairs of oligonucleotide chains respectively, and keep the three pairs of oligonucleotide chains into the f88-4 phage for 2 hours at 42°C, They are named PhA, PhB and PhC respectively.
[0066] 2. Phage transfection of competent Escherichia coli MC1061.
[0067] The MC1061 Escherichia coli in the MC1061 Escherichia coli culture solution is used for streak culture on the basic medium plate, and incubated at 37°C for 24-36 hours to prepare compe...
Embodiment 2
[0072] Embodiment 2: Recombinant expression of FSHR polypeptide
[0073] After knowing the nucleotide sequence (SEQ ID No.1, SEQ ID No.3 and SEQ ID No.5) disclosed in the present invention for encoding a specific FSHR polypeptide, this nucleotide can be used to encode other proteins (such as host The nucleotides of the protein or its fragments) are linked together to express the fusion protein. There are many vectors used to express proteins, and the expression methods are also known to those of ordinary skill in the art, and will not be described here one by one.
[0074] The DNA encoding the specific FSHR polypeptide disclosed in the present invention can be transfected into eukaryotic cells, such as human and other mammalian cells. The transfection method has become a standard method and will not be described here.
Embodiment 3
[0075] Embodiment 3: the preparation of pharmaceutical composition
[0076]A therapeutically effective amount of the FSHR polypeptide of the present invention (SEQ ID No.2, SEQ ID No.4 or SEQ ID No.6) or its expression vector (including but not limited to plasmid, phage, Escherichia coli or mammalian cells, the following same), or the nucleotide encoding the FSHR polypeptide (SEQ ID No.1, SEQ ID No.3 or SEQ ID No.5) or its expression vector alone or mixed with each other to form a pharmaceutical composition with pharmaceutically acceptable adjuvants , the pharmaceutical composition can be used alone or mixed with other drugs that do not affect the function of the pharmaceutical composition. The preparation of these pharmaceutical compositions can be in any pharmaceutically acceptable dosage form, including but not limited to tablets, pills, capsules, injections, oral liquids or liposomes. Methods for the preparation of these formulations are well known to those of ordinary sk...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com