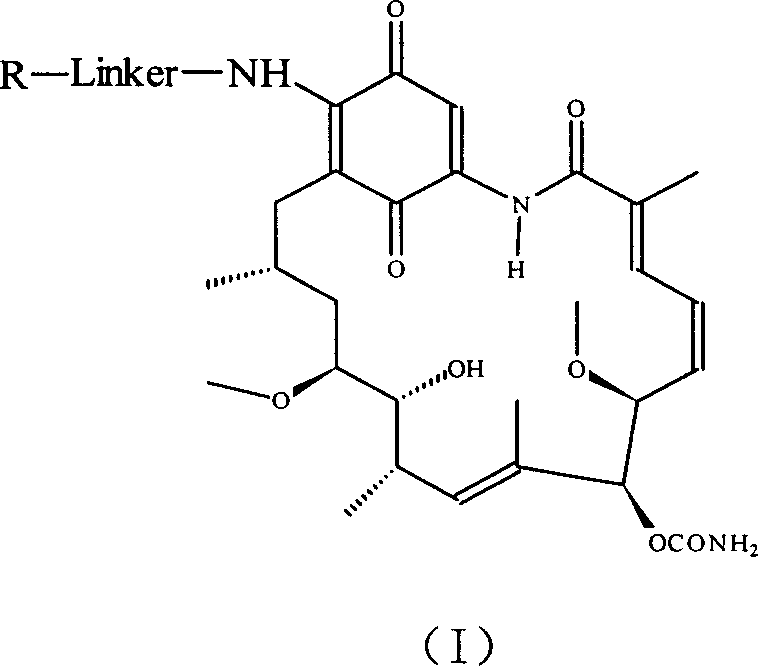
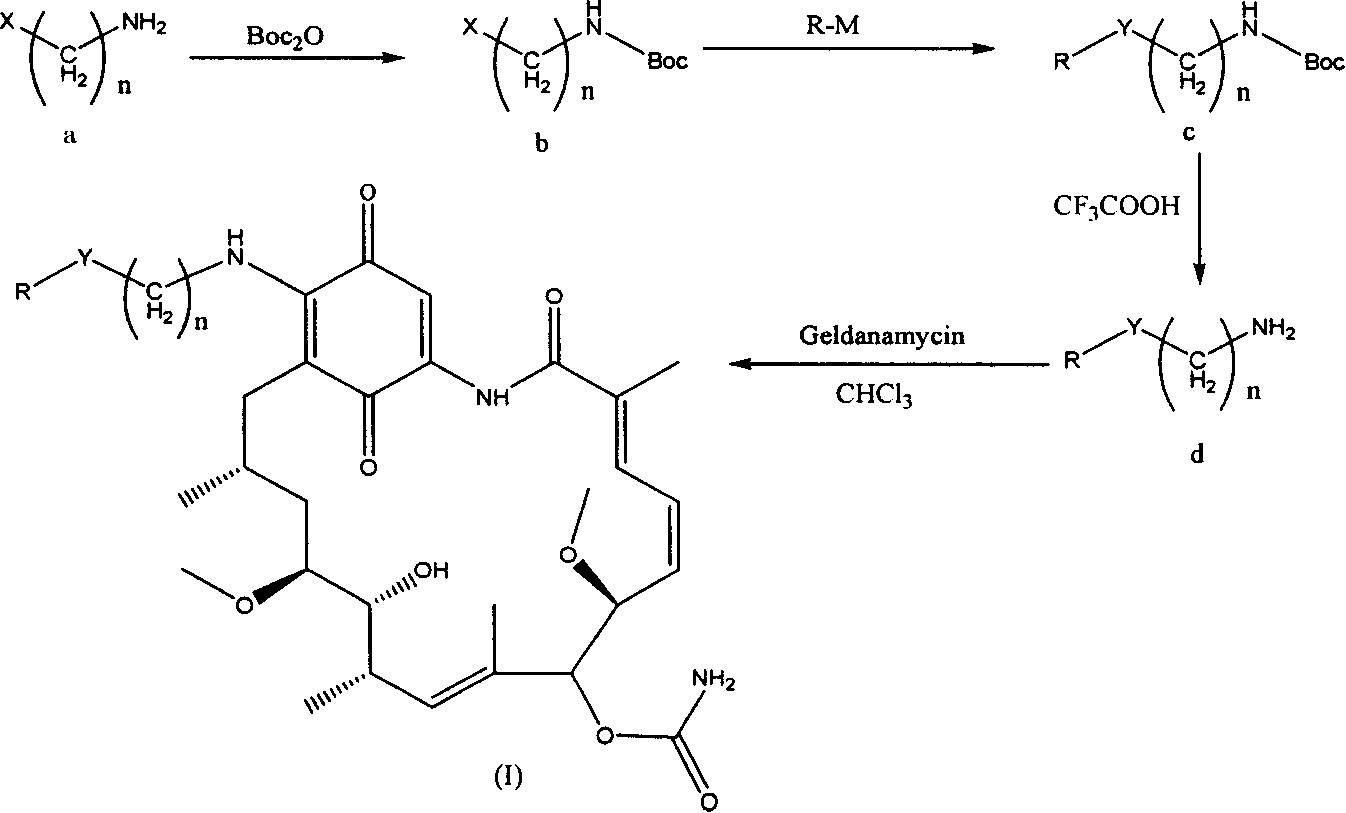
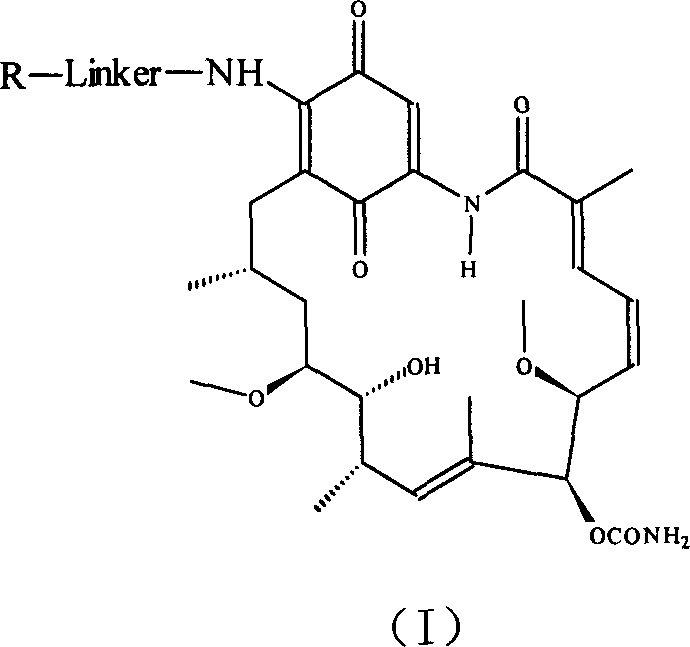
Group of geldanamycin derivative with nucleoside base
A geldanamycin and nucleoside base technology, applied in the field of geldanamycin derivatives, can solve problems such as no literature reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: 17-(4'-((5"-(4-amino-2-oxopyrimidin-1(2H)-yl)-1",3"-oxathiolane-2" Preparation of -yl)methoxy)-4'-oxobutylamine)-17-desmethoxygeldanamycin (1)
[0040] With reference to the literature (Zhao Zhizhong, Protecting Groups in Organic Chemistry, Science Press, 1984: 41-49), use Boc 2 O is used as a raw material to protect the primary amino group of γ-aminobutyric acid to obtain γ-tert-butoxyamide-butyric acid.
[0041] Take 0.45g (2.22mmol) of γ-tert-butoxyamide-butyric acid, add 5mL CHCl 3 . After dissolving, 0.6 g (2.91 mmol) of dicyclohexylcarbodiimide (DCC) was added and stirred at room temperature, a large amount of white turbidity appeared. The reaction was carried out for 4 hours, the white precipitate was filtered off, and the filtrate containing γ-tert-butyroxyamide-butyric anhydride was set aside.
[0042] Take 0.6g (2.63mmol) of lamivudine and place it in a 250mL round-bottomed flask with a reflux condenser, add 50mL of N,N-dimethylformamide, and ...
Embodiment 2
[0047] Example 2: 17-(4'-((5"-(4-amino-5-fluoro-2-oxopyrimidin-1(2H)-yl)-1",3"-oxathia Preparation of cyclopent-2"-yl)methoxy)-4'-oxobutylamine)-17-desmethoxygeldanamycin (2)
[0048] The nucleobase part uses 5-flulamivudine as the raw material, and the Linker part is γ-aminobutyric acid, which is synthesized by a method similar to Example 1 to obtain (2).
[0049] 1 H-NMR δ(ppm): 0.9~1.3(m, 6H, C 10 -CH 3 , C 14 -CH 3 ); 1.3~1.4(m, 5H, C 13 -H 2 , C 14 -H,C 15 -H 2 ); 1.5~1.7(m, 2H, C 17 -NH-CH 2 -CH 2 -CH 2 -COO); 1.983(s, 3H, C 8 -CH 3 ); 2.211(s, 3H, C 2 -CH 3 ); 2.3~2.4(m, 1H, C 10 -H); 2.632(s, 2H, CH 2 -COO); 2.8~3.0(m, 4H, C 17 -NH-CH 2 , oxathione 4-position 2H); 3.182(s, 3H, C 12 -OCH 3 ); 3.458(s, 3H, C 6 -OCH 3 ); 3.5~3.7(m, 4H, C 11 -H,C 12 -H); 4.2~4.3(m, 2H, COO-CH 2 -CH-S); 4.6~4.7(m, 1H, C 6 -H); 5.294(s, 1H, C 7 -H); 5.3~5.4(m, 1H, 2-position 1H of oxathione ring); 5.9~6.2(m, 2H, C 9 -H,C 5 -H); 6.427(s, 1H, 5-position 1H of...
Embodiment 3
[0051] Example 3: 17-(4'-(2"-((2-amino-6-oxygen-1,6-dihydro-4H-purin-9(5H)-yl)methoxy )ethoxy)-4'-oxobutylamine)-17-desmethoxy-geldanamycin (3)
[0052] Acyclovir is used as the raw material for the nucleoside base part, and gamma-aminobutyric acid is used for the Linker part, which is synthesized by a method similar to Example 1 to obtain (3).
[0053] 1 H-NMR δ(ppm): 0.7~1.1(m, 6H, C 10 -CH 3 , C 14 -CH 3 ); 1.2~1.5(m, 5H, C 13 -H 2 , C 14 -H,C 15 -H 2 ); 1.645(m, 2H, C 17 -NH-CH 2 -CH 2 -CH 2 -COO); 2.135(s, 3H, C 8 -CH 3 ); 2.261(s, 3H, C 2 -CH 3 ); 2.3~2.4(m, 1H, C 10 -H); 2.6~2.7(m, 2H, CH 2 -COO); 2.7~2.9(m, 2H, C 17 -NH-CH 2 ); 3.242(s, 3H, C 12 -OCH 3 ); 3.388(s, 3H, C 6 -OCH 3 ); 3.5~3.9(m, 6H, C 11 -H,C 12 -H, nucleoside side chain O-CH 2 -CH 2 OOC); 4.1~4.4(m, 2H, nucleoside side chain OCH 2 -CH 2 -OOC); 4.6~4.8(m, 1H, C 6 -H); 5.073(s, 1H, C 7 -H); 5.283(s, 2H, nucleoside side chain N-CH 2 -O); 6.0~6.3(m, 2H, C 9 -H,C 5 -H); ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com