Method for direct hydrothermal synthesis of chabasite with intermediate silica-alumina ratio
A technology for hydrothermal synthesis and aluminosilicate zeolite, which is applied in the directions of crystalline aluminosilicate zeolite, chemical instruments and methods, molecular sieve catalysts, etc. handy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 8.0 grams of sodium hydroxide, 13.2 grams of potassium hydroxide (85%) and 150 grams of deionized water were prepared into a solution, and 6.2 grams of ammonium oxalate was added and stirred to dissolve. Put in 2.7 grams of aluminum powder to dissolve. After the solution temperature is down to room temperature, add 24.0 grams of white carbon black, stir to obtain hydrogel, the molar ratio of the material for synthesizing hydrogel is 0.5AOxa (ammonium oxalate): 2.0Na 2 O:2.0K 2 O: Al 2 o 3 :8SiO2 2 : 150H 2 O. It was left to stand at room temperature for 2 hours, then transferred to a Teflon-lined stainless steel sealed kettle, heated to 110°C, and left to stand for 2 days. Then cool to room temperature, open the sealed kettle, pour off the supernatant, recover the white solid, and wash it three times with clear water. The resulting white powder was dried at 80°C.
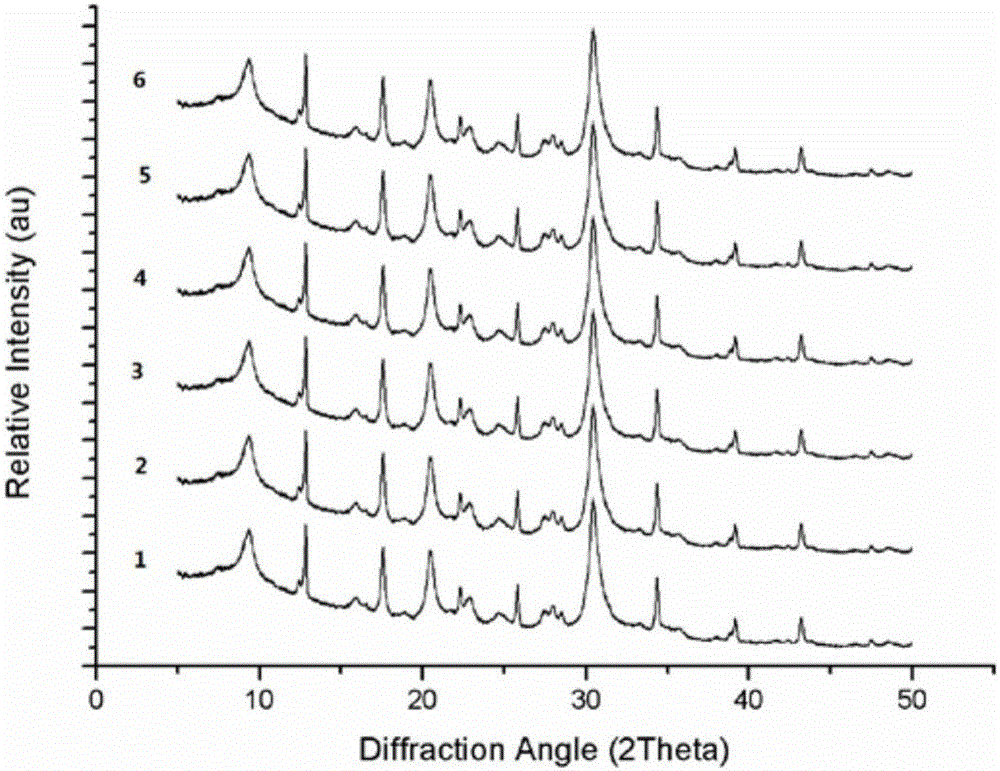
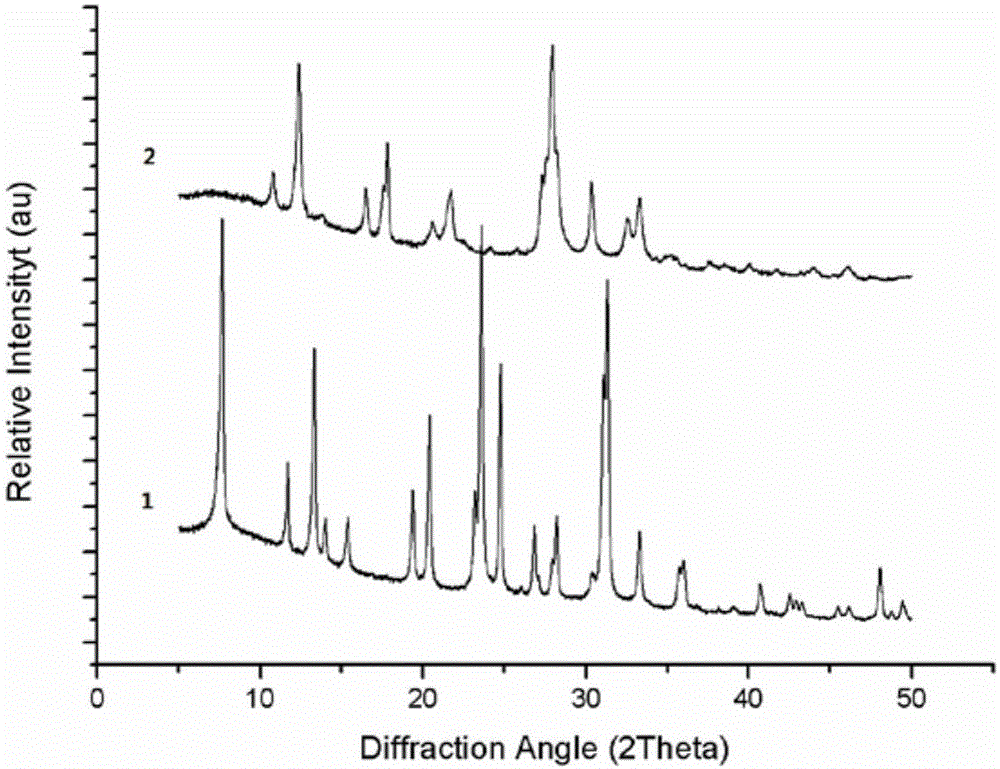
[0029] attached Picture 1-1 It is the powder X-ray diffraction pattern of the product obtained i...
Embodiment 2
[0032] Dissolve 4.0 grams of sodium hydroxide and 19.7 grams of 85% potassium hydroxide in 45.0 grams of deionized water, add 4.6 grams of ammonium acetate, and add 6.66 grams of aluminum sulfate octadecahydrate to dissolve. Then slowly add 60.0 grams of 40% silica sol while stirring, stir to obtain hydrogel, the molar ratio of the material for synthesizing hydrogel is 6.0AAc (ammonium acetate): 10.0Na 2 O:30.0K 2 O: Al 2 o 3 :80SiO2 2 : 800H 2 O. After standing at room temperature for 12 hours, the aged mixture was sealed in a Teflon-lined stainless steel kettle, heated to 150 °C, and allowed to stand for 5 days. Cool to room temperature, open the sealed kettle, pour off the supernatant, recover the white solid, and wash with water three times. The resulting white powder was dried at 80°C.
[0033] attached Figure 1-2 It is the powder X-ray diffraction pattern of the product obtained in this embodiment, and it can be known that the product is a zeolite molecular siev...
Embodiment 3
[0035]Dissolve 2.65 grams of potassium hydroxide in 12.0 grams of deionized water, add 0.8 grams of ammonium bicarbonate, and add 0.82 grams of sodium metaaluminate to dissolve. Slowly add 7.5 grams of 40% silica sol under stirring, stir to obtain hydrogel, the molar ratio of the material for synthesizing hydrogel is 2.0AHC (ammonium bicarbonate): 1.0Na 2 O:4.0K 2 O: Al 2 o 3 :10SiO2 2 :190H 2 O. After the mixture was stirred evenly, without aging, it was directly transferred to a stainless steel kettle with a Teflon liner, heated to 100 degrees Celsius, allowed to stand for 3 days, then cooled to room temperature, the sealed kettle was opened, and the supernatant liquid was poured out. The white solid was recovered and washed three times with water. The resulting white powder was dried at 80°C.
[0036] attached Figure 1-3 It is the powder X-ray diffraction pattern of the product obtained in this embodiment, and it can be known that the product is a zeolite molecular...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com