Kangweisu granular preparation and its preparation method
A granule preparation, kangweisu technology, applied in the field of medicine, can solve the problems of difficult preparation of granules, complicated process screening, etc., and achieve the effects of strong compliance, good taste, and strong hygroscopicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
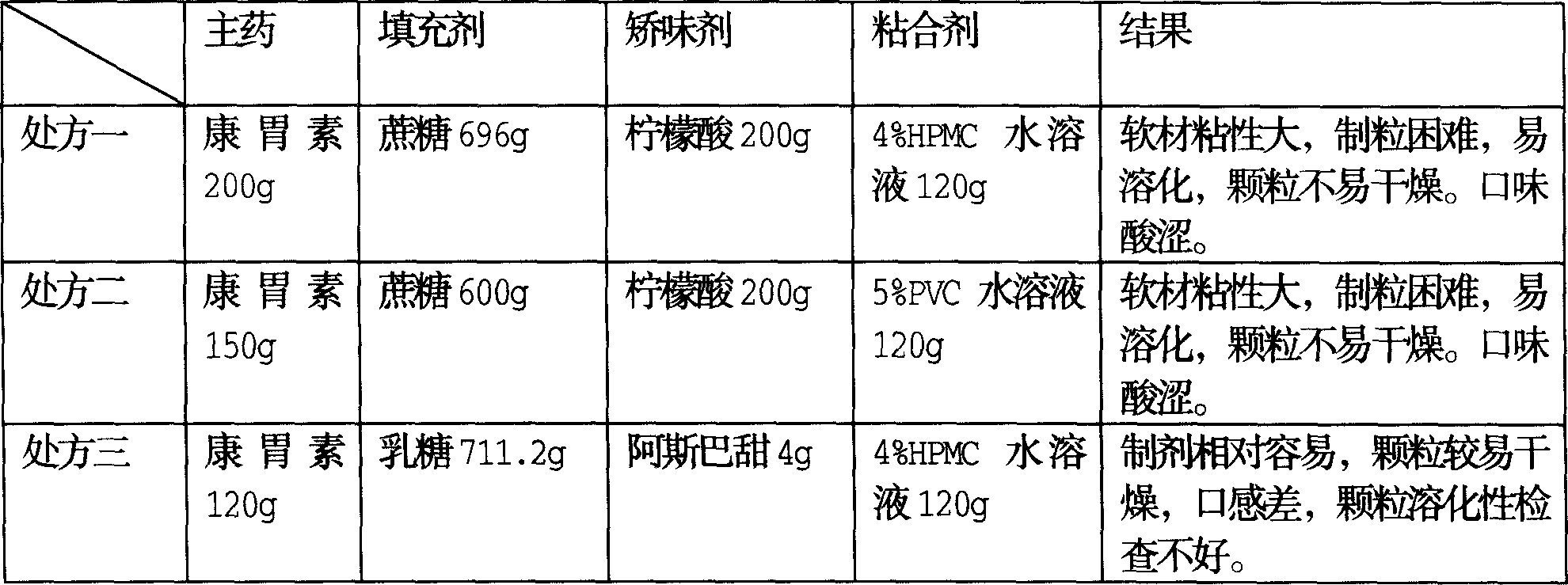
[0015] Aiming at the problem of kangweisu's strong hygroscopicity and difficulty in preparing granular dosage forms, the present invention carried out a series of tests on different proportions of main ingredients and auxiliary materials. The test results are shown in Table 1:
[0016] Table 1: Selection table of main and auxiliary materials for prescription
[0017]
[0018] Prescription four
Kangweisu
110g
Lactose 745g
Mannitol 150g
150g)
Aspartame 6g
4% HPMC water soluble
130g of liquid
The preparation is easy, the granules are easy to dry, and the mouth
Feel better, the particle solubility check is not
Great.
Prescription five
Kangweisu
100g
Lactose 745g
Mannitol 150g
(Mannitol
150g)
Aspartame 6g
8% soluble lake
Powder solution
100g
The preparation is easy, the granules are easy to dry, and the mouth
It feels good, and the particle melting property is qualified.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com