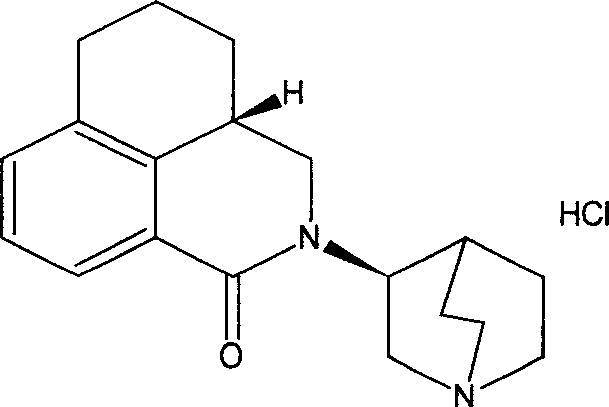
Stable palonosetron injection liquid and its preparation method
A technology of palonosetron and injection, which is applied in the field of drug intravenous injection and can solve the problems of insufficient stability of palonosetron hydrochloride solution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Palonosetron hydrochloride injection formula (prescription) is shown in Table 1
[0033] prescription ingredients
1
2
3
4 (commercial prescription)
palonox hydrochloride
Joan
Sodium chloride
EDTA
Sodium citrate
PH value
Add water for injection to
0.28mg
250mg
-
-
6.4mg
5mg
3.73
5ml
0.28mg
-
250mg
-
6.4mg
5mg
3.75
5ml
0.28mg
-
-
45mg
6.4mg
5mg
3.75
5ml
0.28mg
207mg
2.5mg
7.8mg
18mg
5.10
5ml
[0034] Preparation Process:
[0035] Weigh xylitol, citric acid, and trisodium citrate according to the amount of prescription 1, add an appropriate amount of water for injection to dissolve, add an appropriate amount of activated carbon for needles, heat...
Embodiment 2
[0039] Stability test
[0040]Palonosetron hydrochloride injection samples (prescription 1, 2, 3,) and foreign commercially available injection formulation samples (prescription 4) prepared in Example 1 were placed in a 60°C influencing factor oven, placed for 10 days, Observe and compare their appearance and changes in the amount of impurities on the 0th day, the 5th day and the 10th day, and investigate the thermal stability of the preparation. The related substances on the 0th day, the 5th day and the 10th day were determined by high performance liquid chromatography. The results showed that compared with foreign commercially available products, the injection samples prepared in Example 1 had no obvious changes in appearance and impurity amount after being placed at 60°C for 5 days and 10 days, indicating that they had better thermal stability. See Table 2 for specific data.
[0041] prescription number
[0042] Conclusion: adopt the injection sample prepared by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com