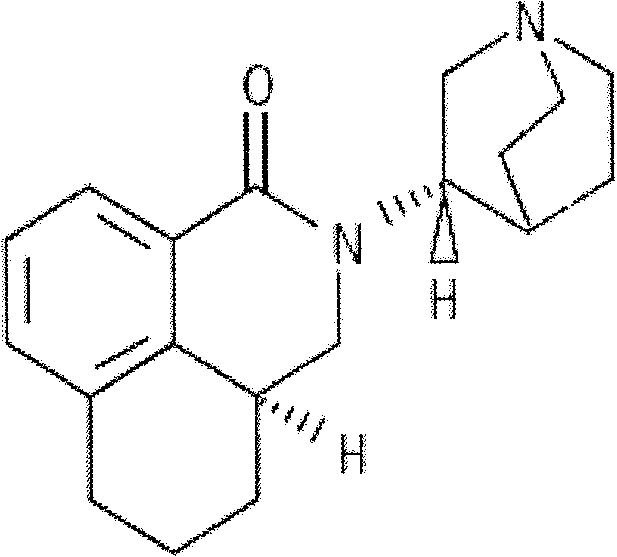
Safe and stable palonosetron injection
A technology of palonosetron and composition, which is applied in the direction of drug combination, medical preparations containing active ingredients, digestive system, etc. It can solve the problems of poor stability and unqualified color, and achieve the effect of low toxicity and small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
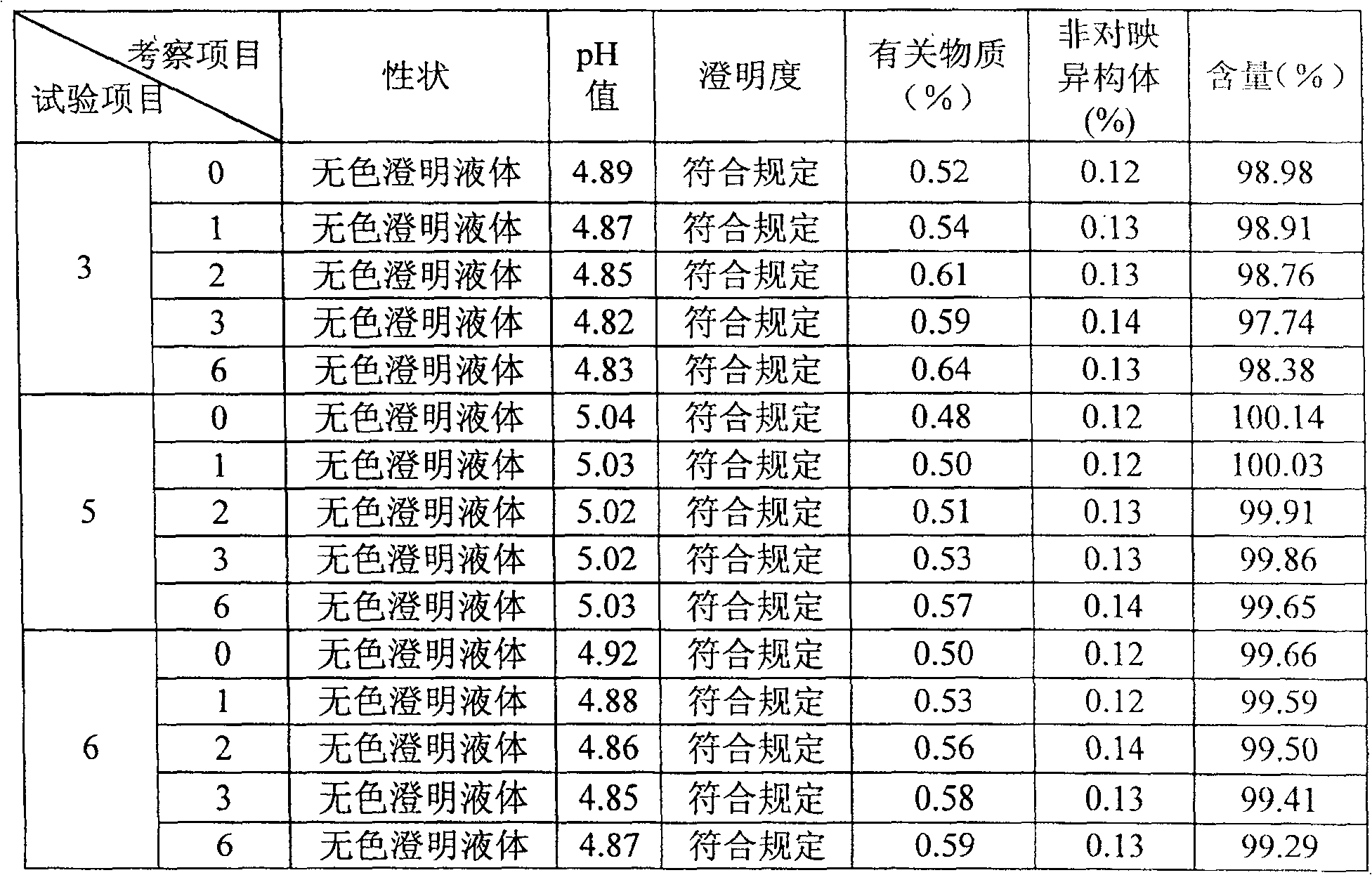
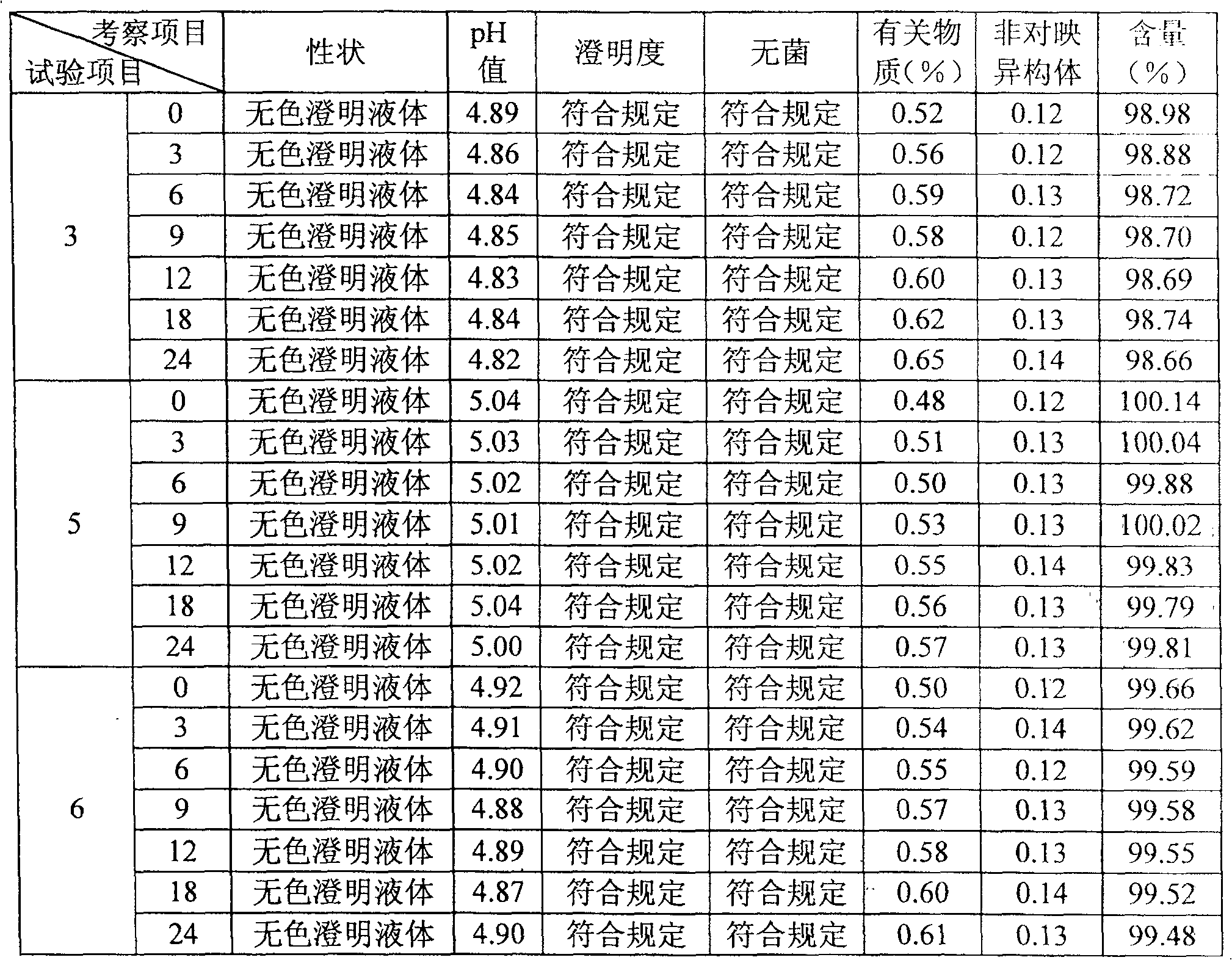
[0033] Embodiment 1 Palonosetron Hydrochloride Injection
[0034] Preparation prescription (based on 1000ml):
[0035] Palonosetron Hydrochloride 0.0112g (contains Palonosetron 0.01g)
[0036] Sodium citrate 1g
[0037] Glucose 60g
[0038] Calcium Disodium EDTA 0.1g
[0039] Proper amount of dilute hydrochloric acid
[0040]
[0041] Add water for injection to 1000ml
[0042] It is prepared according to the following steps: dissolve sodium citrate, glucose and calcium disodium EDTA in water for injection and dilute appropriately, adjust the pH of the solution to 4.5 with dilute hydrochloric acid, and then add activated carbon for adsorption. After coarse filtration, add the prescribed amount of palonosetron hydrochloride to dissolve and stir evenly to replenish the water for injection to the full amount. After filtering, test the content and pH, fill and seal after passing the test, and sterilize.
Embodiment 2
[0043] Embodiment 2 Palonosetron Hydrochloride Injection
[0044] Preparation prescription (calculated in 1000ml)
[0045] Palonosetron Hydrochloride 0.5615g (contains Palonosetron 0.5g)
[0046] Sodium dihydrogen phosphate 50g
[0048] Calcium Disodium EDTA 0.75g
[0049] Appropriate amount of sodium hydroxide
[0050]
[0051] Add water for injection to 1000ml
[0052] It is prepared according to the following steps: dissolve and dilute the prescription amount of sodium dihydrogen phosphate, sodium chloride and calcium disodium EDTA with water for injection, adjust the pH of the solution to 5.5 with sodium hydroxide, and then add activated carbon for adsorption. After coarse filtration, add the prescribed amount of palonosetron hydrochloride to dissolve and stir evenly to replenish the water for injection to the full amount. After filtering, test the content and pH, fill and seal after passing the test,...
Embodiment 3
[0053] Embodiment 3 palonosetron hydrochloride injection
[0054] Preparation prescription (calculated in 1000ml)
[0055] Palonosetron Hydrochloride 0.0562g (contains Palonosetron 0.05g)
[0056] Citric acid 20g
[0057] Mannitol 41.5g
[0058] Calcium Disodium EDTA 0.5g
[0059] Appropriate amount of sodium hydroxide
[0060]
[0061] Add water for injection to 1000ml
[0062] It is prepared according to the following steps: dissolving and properly diluting the prescribed amount of citric acid, mannitol and calcium disodium EDTA with water for injection, adjusting the pH of the solution to 5.5 with sodium hydroxide, and then adding activated carbon for adsorption. After coarse filtration, add the prescribed amount of palonosetron hydrochloride to dissolve and stir evenly to replenish the water for injection to the full amount. After filtering, test the content and pH, fill and seal after passing the test, and sterilize.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com