Injection of palonosetron and preparation process thereof
A technique for palonosetron and injection, which is applied in the field of intravenous injection in which palonosetron hydrochloride is an active ingredient, can solve the problems of stability and high cost, achieve simple and feasible production technology, improve stability, and be easy to use. The effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of Palonosetron Hydrochloride Injection
[0029] (1) Specific research process and experimental results of component screening
[0030] According to the water-soluble nature of palonosetron hydrochloride, water for injection was used as the solvent to prepare the following five components, and the composition of components 3 and 4 was similar to that of commercially available products. The specific composition of components and the results of the investigation of influencing factors are shown in Table 1.
[0031] Table 1 Palonosetron hydrochloride component screening table
[0032]
Components
component 1
component 2
Component 3
Component 4
Component 5
Component 6
(commercial prescription)
Palonosetron hydrochloride (g)
0.28
0.28
0.28
0.28
0.28
0.28
Citric acid (g)
-
25.5
4.2
0.8 ...
Embodiment 2
[0037] Embodiment 2 stability test
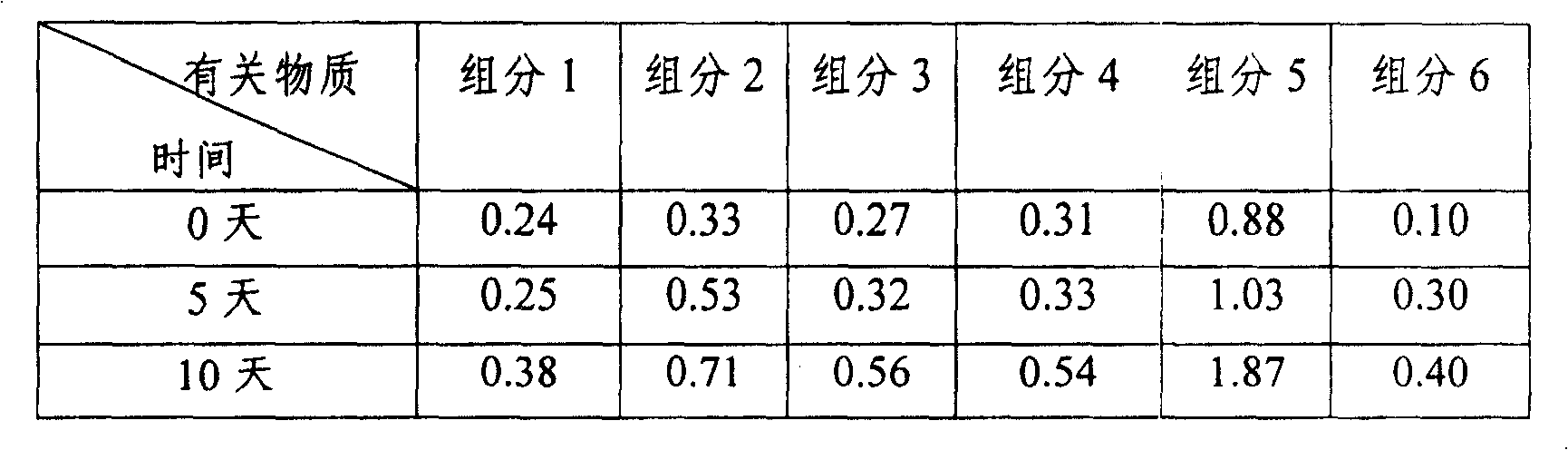
[0038] The palonosetron hydrochloride injection samples (components 1 to 5) and foreign commercially available injection formulation samples (component 6) prepared in Example 1 were placed in an oven with influencing factors at 60°C for 10 days, and observed and compared The changes of their appearance and impurity amount on the 0th day, the 5th day and the 10th day were used to investigate the thermal stability of the preparation. The related substances on the 0th day, the 5th day and the 10th day were determined by high performance liquid chromatography. The results showed that, compared with the commercially available products, the appearance and amount of impurities of the injection samples prepared in Example 1 had no significant changes after being placed at 60°C for 5 days and 10 days, indicating that they had better thermal stability. See Table 2 for specific data.
[0039] Table 2 The results of the determination of related subst...
Embodiment 3
[0042] Embodiment 3 Pharmacological experiment
[0043] According to the requirements of the "Measures for the Administration of Drug Registration", we have conducted special safety tests on palonosetron hydrochloride injection such as allergy, hemolysis and local irritation.
[0044] In the guinea pig systemic active allergy test (ASA), 24 FMMU guinea pigs were randomly divided into negative control group, positive control group and palonosetron hydrochloride low and high dose groups (0.019 and 0.058mg / kg. bw, the dosage is converted according to 1 and 3 times of the planned dosage for one clinical intravenous injection respectively). Intraperitoneal injection, once every other day, for three consecutive times. Animals in each group were challenged on the 12th day after the last injection. Results The guinea pigs in the negative control group had no obvious allergic reaction symptoms, all the guinea pigs in the positive control group died, and the guinea pigs in the low and h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com