Antidepressant composition containing citalopram and cyclodextrin
A technology of composition and cyclodextrin, which is applied in the field of clathrate oral pharmaceutical composition, can solve the problems of increased production cost, increased impurities, and high requirements for equipment and operators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: Bitterness test
[0034] Add β-cyclodextrin to the 2 mg / ml citalopram hydrobromide solution, so that the molar ratio of β-cyclodextrin to citalopram hydrobromide is 0, 0.5, 1.0, 2.0, 3.0, and carry out the solution for 7 people. Bitterness test, the results are as follows:
[0035] Molar ratio (βCD / citalopram hydrobromide) 0 0.5 1.0 2.0 3.0
[0036] Very weak bitterness 0 0 3 7 7
[0037] Stronger bitterness 0 2 4 0 0
[0038] Strong bitterness 7 5 0 0 0
[0039] When β-cyclodextrin is added to the citalopram hydrobromide solution, the bitterness of the solution decreases with the increase of the amount of cyclodextrin, and when the molar ratio of cyclodextrin to citalopram reaches 2:1, the bitterness decreases very significant.
Embodiment 2
[0040] Embodiment 2: solubility test
[0041] In the presence of cyclodextrin, the water solubility of hydrophobic molecules increases, the dissolution rate and the dissolved amount of active substances both increase, so the change in water solubility is a common method to prove the formation of inclusion complexes.
[0042] Prepare three kinds of solutions A, B, C, wherein A is adding 2g citalopram hydrobromide in 50ml water; B is adding 5.6g β-cyclodextrin in 50ml water; C is adding 2g citalopram hydrobromide in 50ml water Phalopram and 5.6 g β-cyclodextrin. Stir the three solutions at the same time until they reach an equilibrium state. It can be observed that the substances in A and B cannot be completely dissolved, while the solution in C is clear. This phenomenon indicates that clathrates are formed, and the solubility of the active substance and cyclodextrin is increased.
Embodiment 3
[0043] Embodiment 3: inclusion rate test
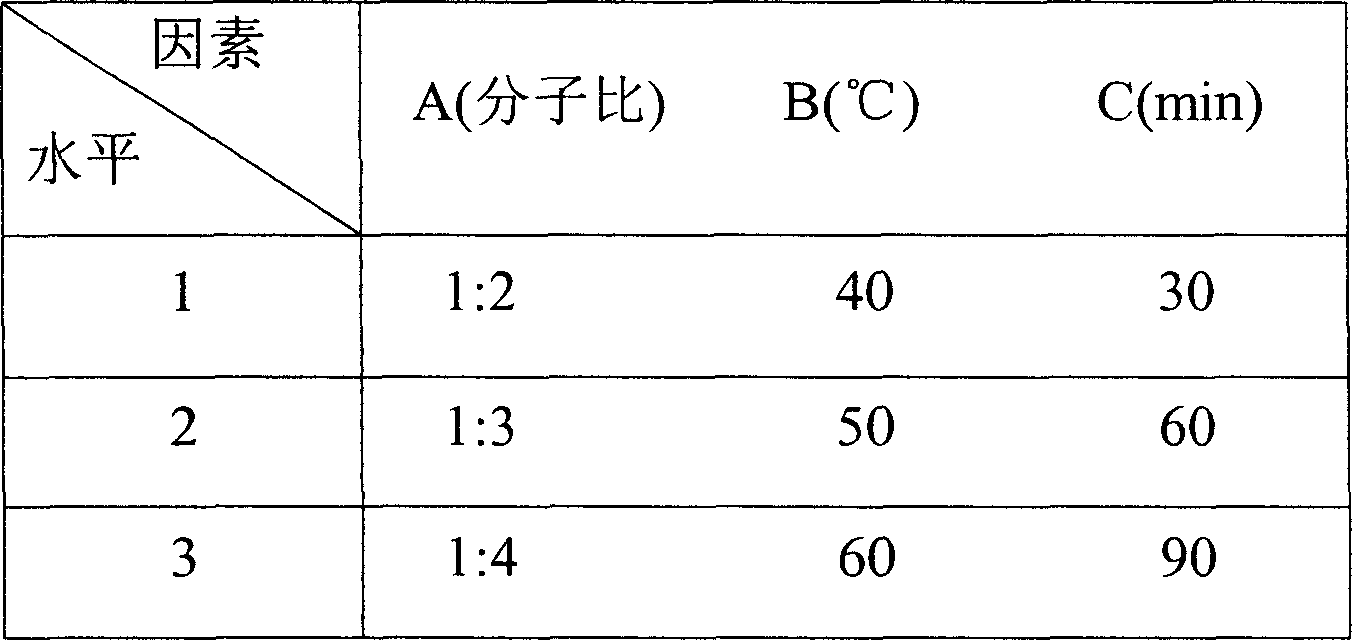
[0044] Select three factors and three levels, and determine the best inclusion process through the following orthogonal experiments (considering the actual production situation, with the inclusion rate as the inspection target), the active substances used in this test are citalopram hydrobromide, cyclodextrin For β-cyclodextrin:
[0045] Inclusion rate = drug content in the inclusion compound / dosage × 100%
[0046] Inclusion compound three-factor three-level table
[0047]
[0048] A: Molecular ratio of drug to cyclodextrin
[0049] B: stirring temperature
[0050] C: Stirring time
[0051] Test No.
[0052] It can be seen from the R value that the influence of each factor on the inclusion rate is B>A>C, and the optimal inclusion process is: A 1 B 2 C 2 , that is, the molecular ratio is 1:2, the inclusion temperature (stirring temperature) is 50° C., and the inclusion time (stirring time) is 60 minutes. Under these...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com