Powder injection of dolasetron and its pharmaceutically acceptable salt, and its preparation method
The technology for dolasetron and preparation is applied in the field of powder injection preparation of dolasetron and its preparation, which can solve the problems of stability of water injection preparation and limitation of transportation and storage conditions, and achieves good stability and long shelf life. , Guaranteed quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
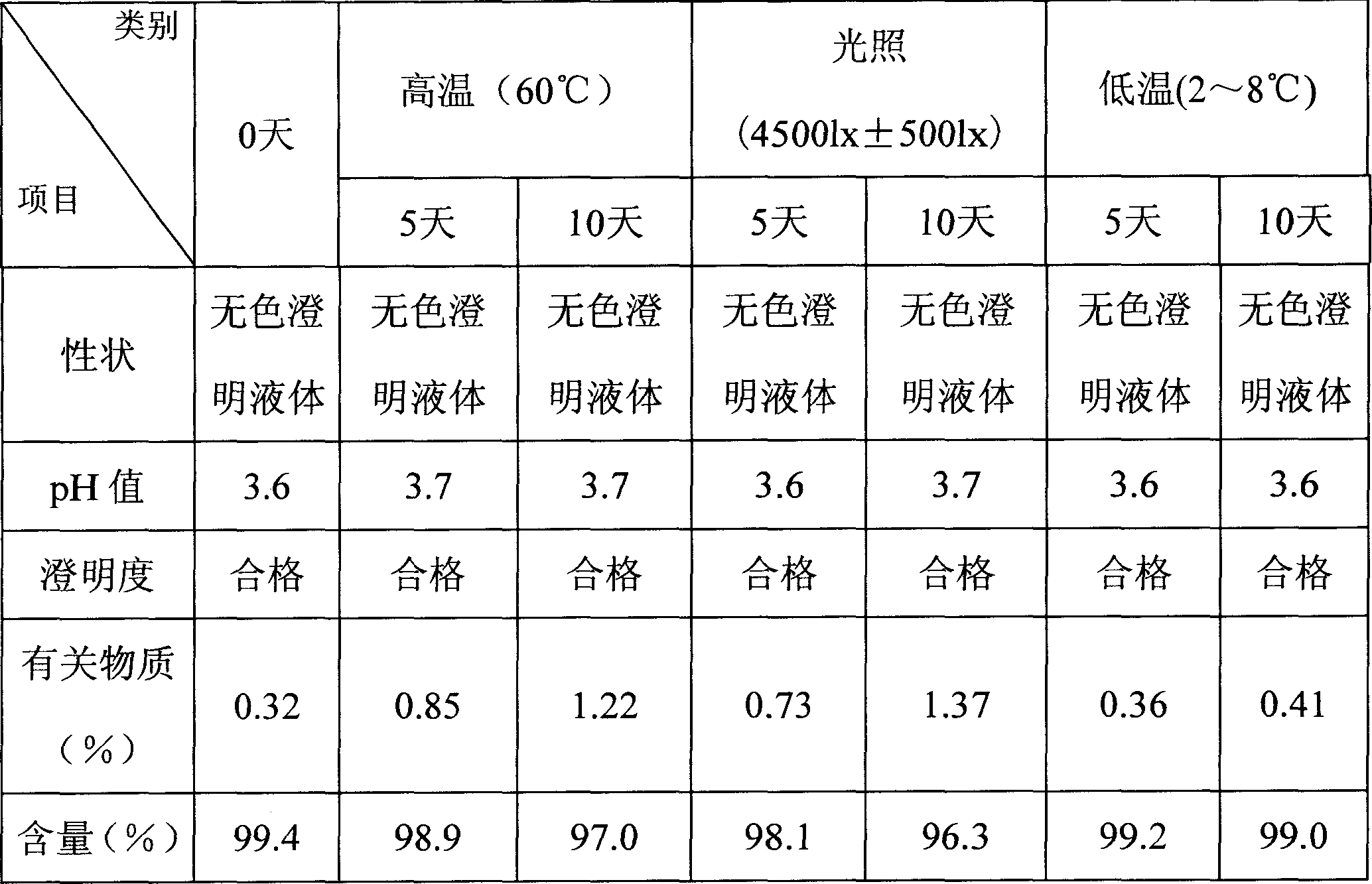
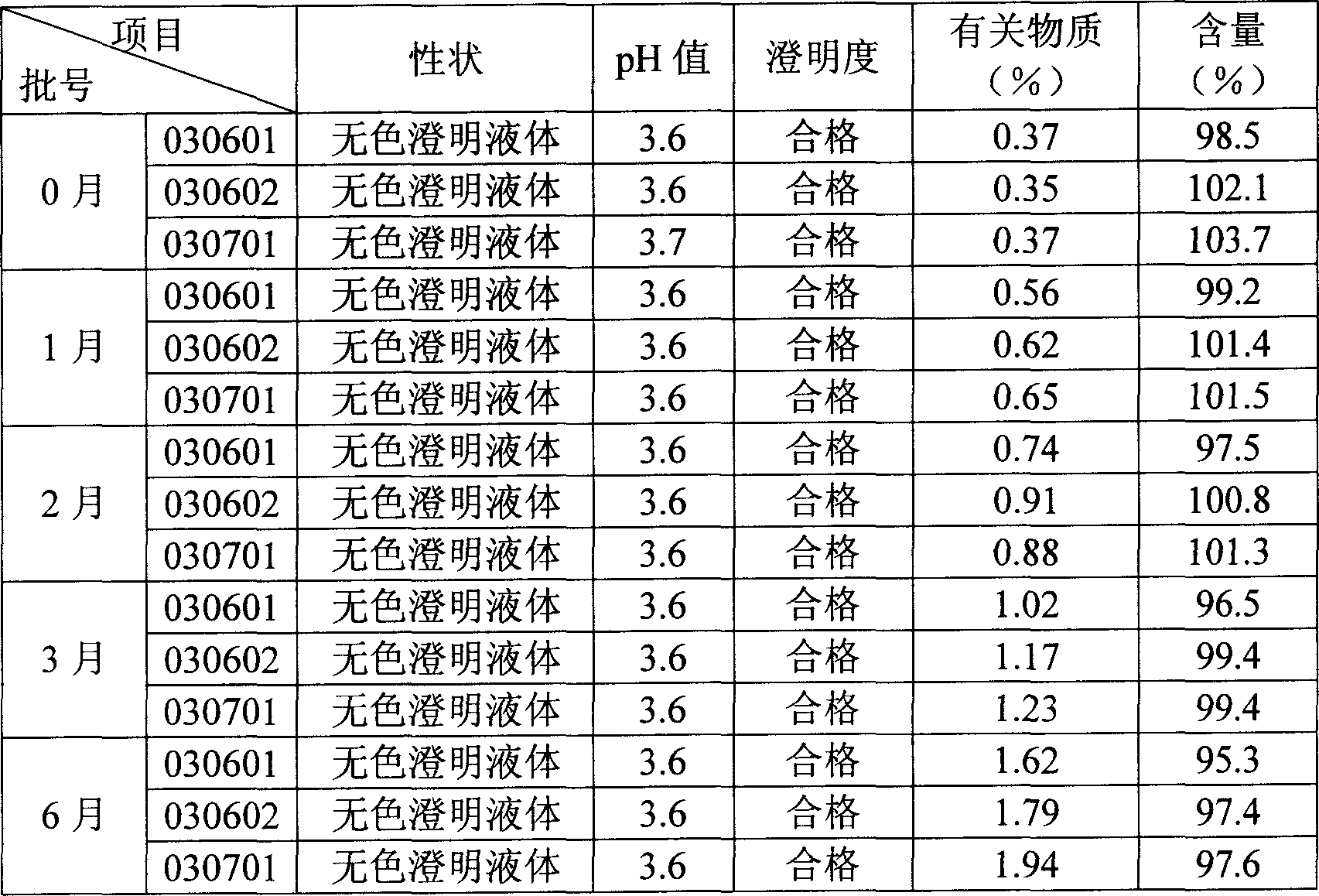
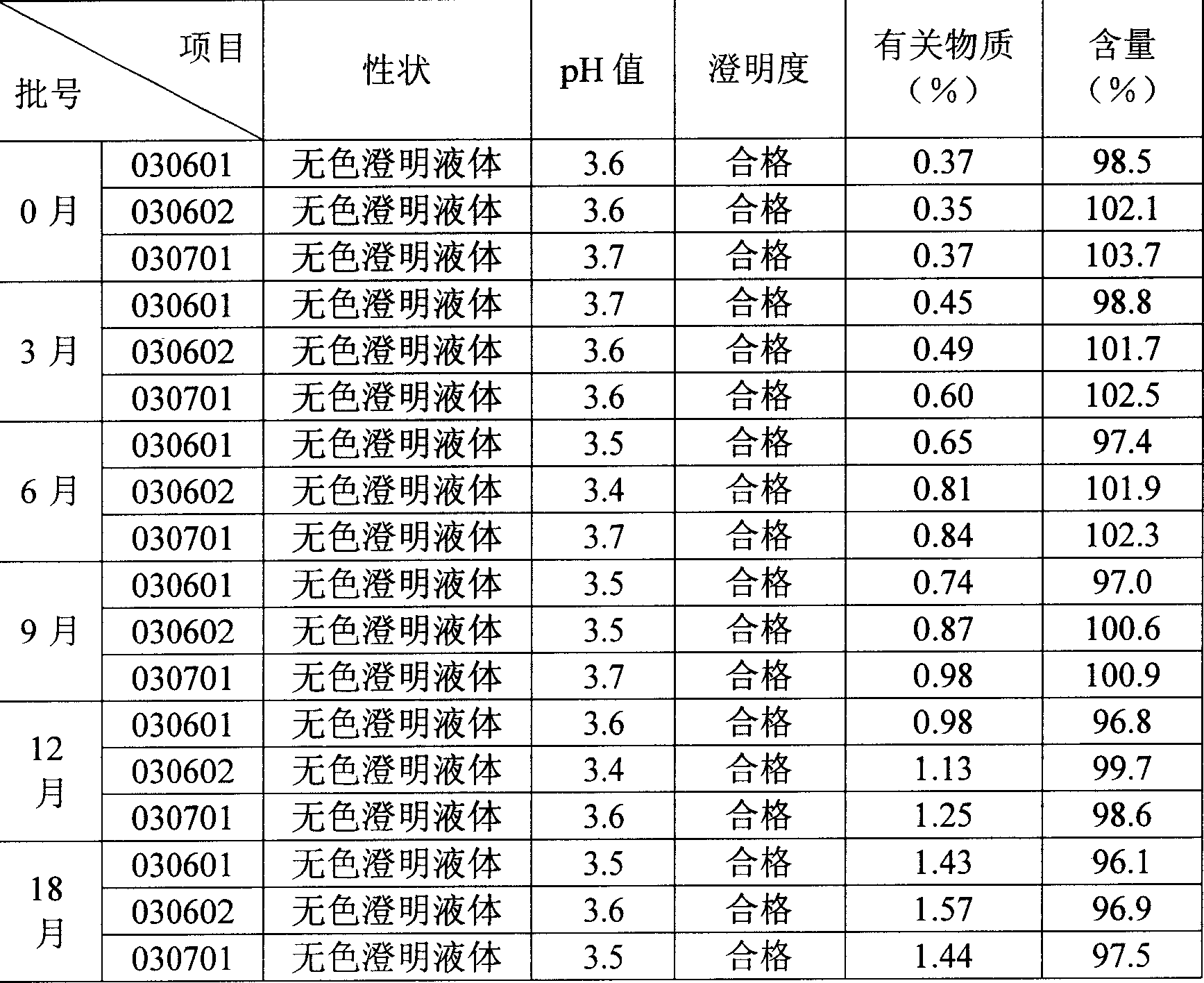
Image
Examples
Embodiment 1
[0071] Embodiment 1: Prescription of dolasetron powder injection preparation (calculated as dolasetron)
[0072] Dolasetron 9.25g
[0073] Mannitol 80g
[0074] Add water for injection to 1000ml
[0075] A total of 1000 pieces were made
[0076] Take the following steps to prepare:
[0077] 1) According to the ingredients of the above preparation prescription, add water for injection of 80wt% of the prescription amount to dissolve;
[0078] 2) Add 0.05wt% activated carbon to the above solution for decolorization, and filter to remove carbon;
[0079] 3) dilute to the preparation amount with the above-mentioned solvent, measure the concentration of the medicinal solution, and the pH value of the medicinal solution is 3.0;
[0080] 4) Filter and sterilize through a filter membrane to a liquid storage tank, sub-package, freeze-dry according to a preset temperature, crimp the cap, and pack.
[0081] The present embodiment adopts general freeze-drying machine freeze-drying: ...
Embodiment 2
[0087] Embodiment 2: Prescription of dolasetron powder injection preparation (calculated as dolasetron)
[0088] Dolasetron 74g
[0089] Mannitol 200g
[0090] Add water for injection to 2000ml
[0091] A total of 1000 pieces were made
Embodiment 3
[0092] Embodiment 3: Prescription of dolasetron powder injection preparation (calculated as dolasetron)
[0093] Dolasetron 74g
[0094] Glycine 180g
[0095] Add water for injection to 2000ml
[0096] A total of 1000 pieces were made
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com