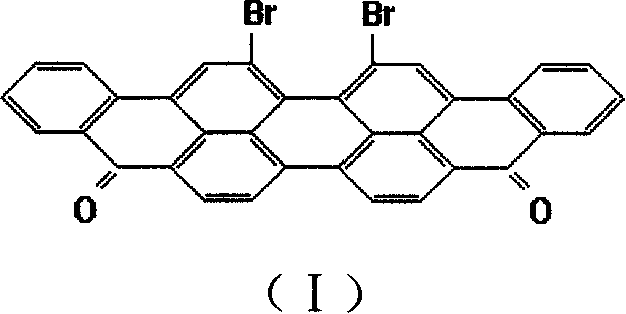
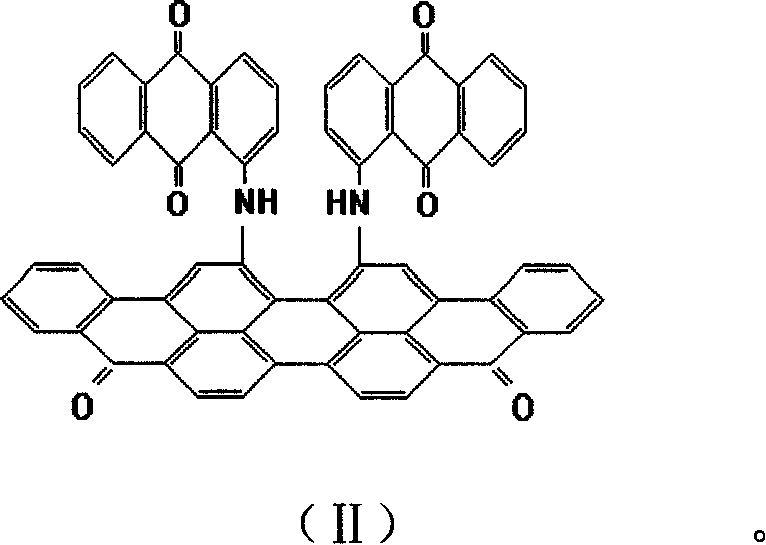
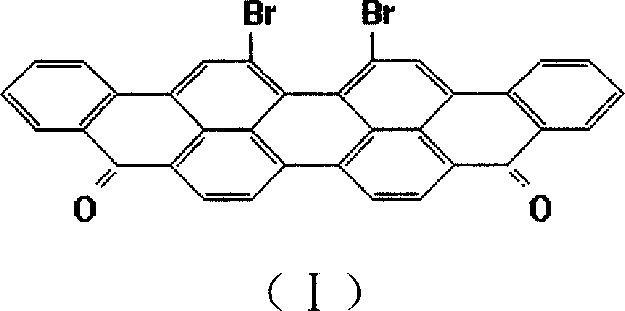
Anthraquinone vat dye 16,17- dibromviolanthrone and 16,17-di(1'-imdoanthraquinonyl) violanthrone and its preparation method
An imino-anthraquinone-based, violinanthrone-based technology, applied in the field of dyes, can solve the problems of unpublished dye structures, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0037] In a 500ml three-necked flask (installed with a thermometer and a reflux condenser, the condenser is filled with frozen brine, with an exhaust pipe and an exhaust gas absorption device attached to remove the generated HCl and SO 2 375g (about 250ml) of 99.5% chlorosulfonic acid, 1.0g of sulfur, and 50g of violinanthrone were added to the acid gas). Start stirring, heat up to 50-70°C, and stir at 50-70°C for 5 hours. The above materials were diluted in a 2000ml beaker with 1000ml of 30% sulfuric acid inside, filtered, washed with water until neutral, and dried to obtain 66.7 g of 16,17-dibromoviolanthrone, with a yield of 99% based on violanthrone. The bromine content of the product was detected to be 26.1% (25.72% in theory). The product is dyed cotton in red light navy blue.
example 2
[0039] Add 16,17-dibromoviolanthrone 15g, 97% 1-aminoanthraquinone 13g, 98% copper chloride 1.5 g in a 2000ml steel solid-phase condenser (attached to a thermometer, vent hole). g, 7.5g of 95% anhydrous sodium carbonate, start the solid-phase condenser, raise the temperature to 100-135°C, keep it for 2 hours, raise the temperature to 230-240°C within 3 hours, keep it for 4 hours, and cool it down to room temperature for 5 hours. Put the above materials into a 1000ml beaker which has been added with 300ml of water and 35g of 30% hydrochloric acid in advance, heat up to 90-95°C, keep for 1 hour, filter, and wash with water until neutral.
[0040]In a 1000ml beaker, add 250ml of water, 25g30% hydrochloric acid, 2g complex complexing agent (EDTA:DEA=1:1) and the above-mentioned material (filter cake), stir at normal temperature for 1 hour, heat up to 80~95°C, keep for 3 hours, Filter, wash with water until neutral, and dry to obtain 21.5g of 16,17-bis(1'-iminoanthraquinone)violant...
example 3
[0042] Add 375 g (about 250 ml) of 99.5% chlorosulfonic acid, 1.0 g iodine, and 50 g violanthrone into a 500 ml three-necked flask. The bromination reaction and treatment were carried out as in Example 1 to obtain 65.3 g of compound 16,17-dibromoviolanthrone similar to Example 1, and the yield was 97% based on violanthrone. The bromine content of the product was detected to be 24.6%. The product is dyed cotton red blue light navy blue.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com