Preparation method of chiral non alpha amino acid derivative of simultaneously protected by hydroxyl group and amino group
A technology for amino acids and derivatives, applied in the field of derivatives synthesis, can solve problems such as the preparation method of undiscovered hydroxyl and amino target chiral compounds, and achieve the effects of high yield, easy reaction, and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
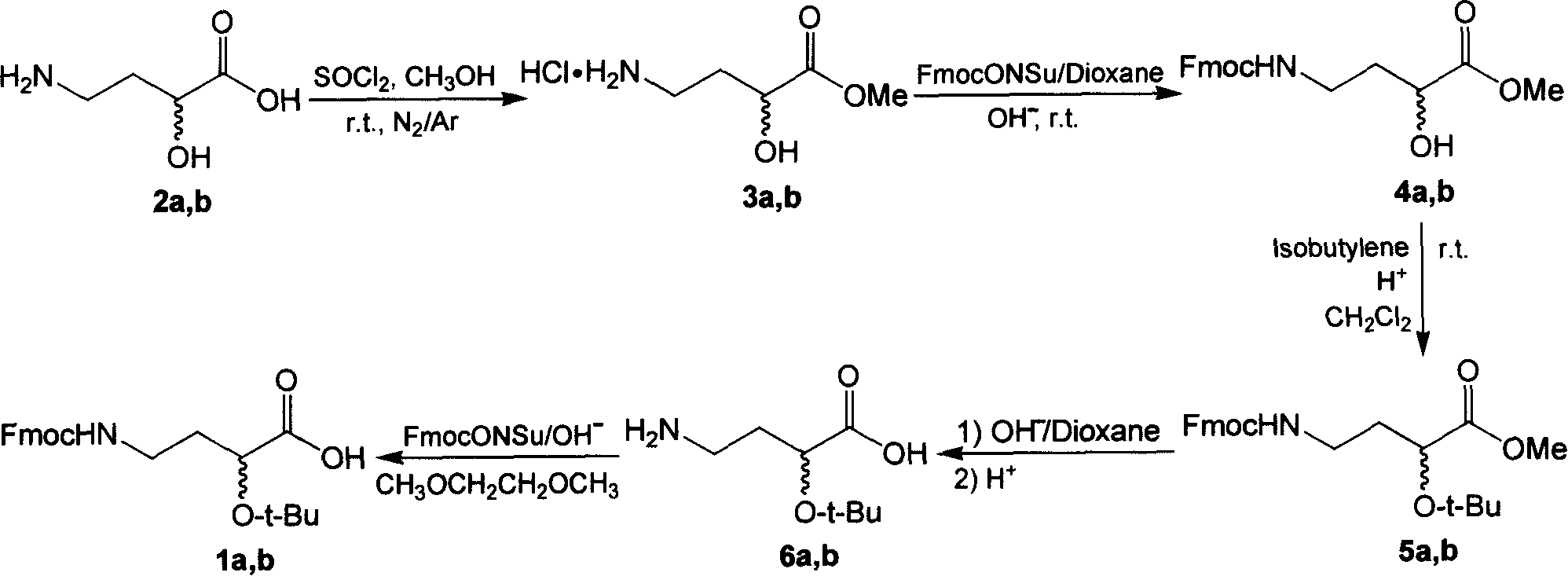
[0037] Example 1: Preparation of (S)-2-tert-butoxy-4-(9-fluorenylmethyloxy) carbonylaminobutyric acid (1a)
[0038] (1) Synthesis of (S)-2-hydroxy-4-aminobutyric acid methyl ester hydrochloride (3a) 5 . Mix 4g (33.58mmol) (S)-2-hydroxy-4-aminobutyric acid 2a, 7mL (95%, 93.35mmol) of thionyl chloride and 60mL methanol at 0°C for 15 minutes, then, at room temperature Continue to stir the reaction until the complete reaction of the raw material (TLC detection, CH 2 Cl 2 / MeOH / HOAc=2 / 2 / 1). Then, the solvent of this reaction mixture was removed by rotary evaporator. Next, after dissolving the obtained solid with a mixed solvent of methanol and carbon tetrachloride (1:1, v / v), the solvent was removed by a rotary evaporator, and the same operation was performed several times. Finally, 5.66 g of white solid product 3a was given (yield: 99.2%). TLC R f 0.50 (CH 2 Cl 2 / MeOH / HOAc, 2 / 2 / 1, v / v). 1 H-NMR (400MHz, D 2 O): δ4.33 (dd, 3 J H,H = 8.4Hz, 3 J H,H =4.0Hz, 1H, 2-H), ...
example 2
[0043] Example 2: Preparation of (R)-2-tert-butoxy-4-(9-fluorenylmethyloxy)carbonylaminobutyric acid (1b)
[0044] Preparation of 1b also adopts the synthetic route of 1a, and the data of each intermediate 3b, 4b, 5b and 6b and the final product 1b are characterized as follows:
[0045] (1) Synthesis of (R)-2-hydroxy-4-aminobutyric acid methyl ester hydrochloride (3b) 5 . white solid. Yield: 97.8%. TLC R f 0.50 (CH 2 Cl 2 / MeOH / HOAc, 2 / 2 / 1, v / v). 1 H-NMR (400MHz, D 2 O): δ4.32 (dd, 3 J H,H = 8.4Hz, 3 J H,H =4.0Hz, 1H, 2-H), 3.67(s, 3H, 1-OCH 3 ), 3.22(d, 3 J H,H = 1.2Hz, 1H, 2-OH), 3.04(m, 2H, 4-H), 2.07 & 1.90(m, 2H, 3-H). ESI Mass: C 5 h 12 ClNO 3 m / z, 169.15; found, 133.10 [M-HCl+H] + .
[0046] (2) Synthesis of (R)-methyl 2-hydroxy-4-(9-fluorenylmethyloxy)carbonylaminobutyrate (4b). white solid. Yield: 78.1%. TLC R f 0.30 (CH 2 Cl 2 / MeOH, 19 / 1, v / v). Analytical HPLC: t R =22.70min. 1 H-NMR (400MHz, DMSO-d 6 ): δ7.87(d, 3 J H,H =7.2Hz, 2H, Fl...
example 3
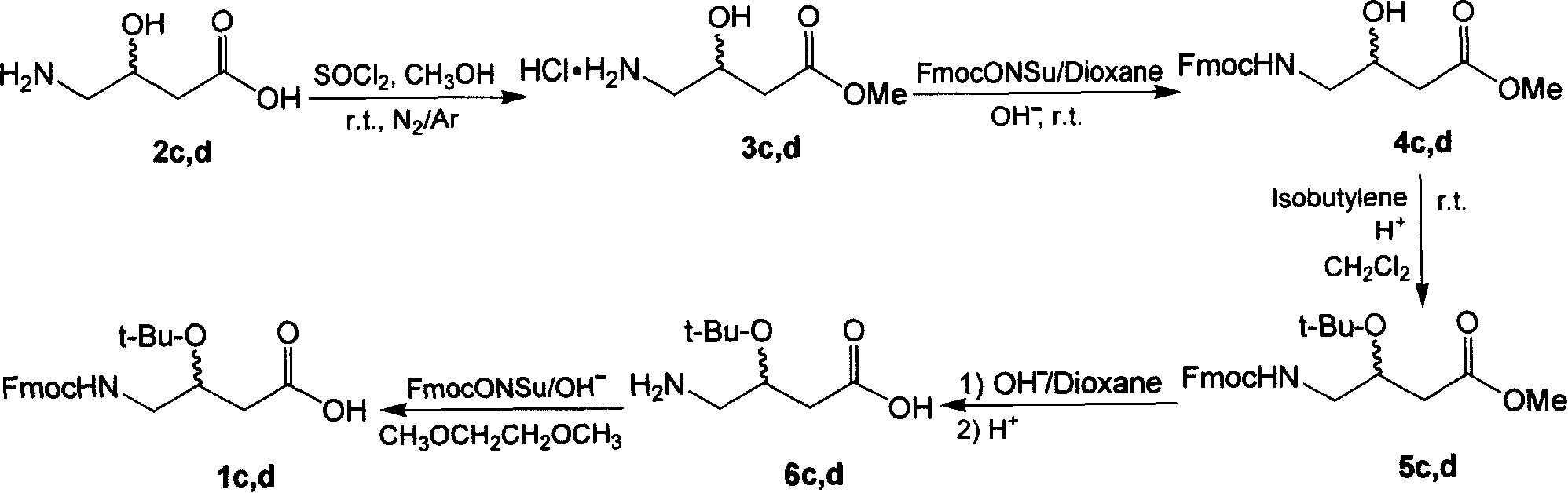
[0050] Example 3: Preparation of (S)-3-tert-butoxy-4-(9-fluorenylmethyloxy)carbonylaminobutyric acid (1c)
[0051] Using synthetic route II completely similar to synthetic route I, 1c can be prepared; the data of each intermediate 3c, 4c, 5c and 6c and the final product 1c are characterized as follows:
[0052] (1) Synthesis of (S)-3-hydroxy-4-aminobutyric acid methyl ester hydrochloride (3c). white solid. Yield: 98.0%. TLC R f 0.52 (CH 2 Cl 2 / MeOH / HOAc, 2 / 2 / 1, v / v). 1 H-NMR (400MHz, D 2 O): δ4.28(m, 1H, 3-H), 3.64(s, 3H, 1-OCH 3 ), 3.35(d, 3 J H,H = 1.6Hz, 1H, 2-OH), 3.10(m, 2H, 4-H), 2.28 & 2.11(m, 2H, 2-H). ESI Mass: C 5 h 12 ClNO 3 m / z, 169.15; found, 133.15 [M-HCl+H] + .
[0053] (2) Synthesis of (S)-methyl 3-hydroxy-4-(9-fluorenylmethyloxy)carbonylaminobutyrate (4c). white solid. Yield: 75.0%. TLC R f 0.32 (CH 2 Cl 2 / MeOH, 19 / 1, v / v). Analytical HPLC: t R =22.72min. 1 H-NMR (400MHz, DMSO-d 6 ): δ7.88(d, 3 J H,H =7.6Hz, 2H, Fluoren.-H), 7.69(...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com