Method for preparing lithium chloride free from water
A technology of lithium chloride and calcium chloride, which is applied in the direction of lithium halide, etc., can solve the problems of immaturity, and achieve the effect of simplifying the operation steps, reducing energy and water consumption, and shortening the process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
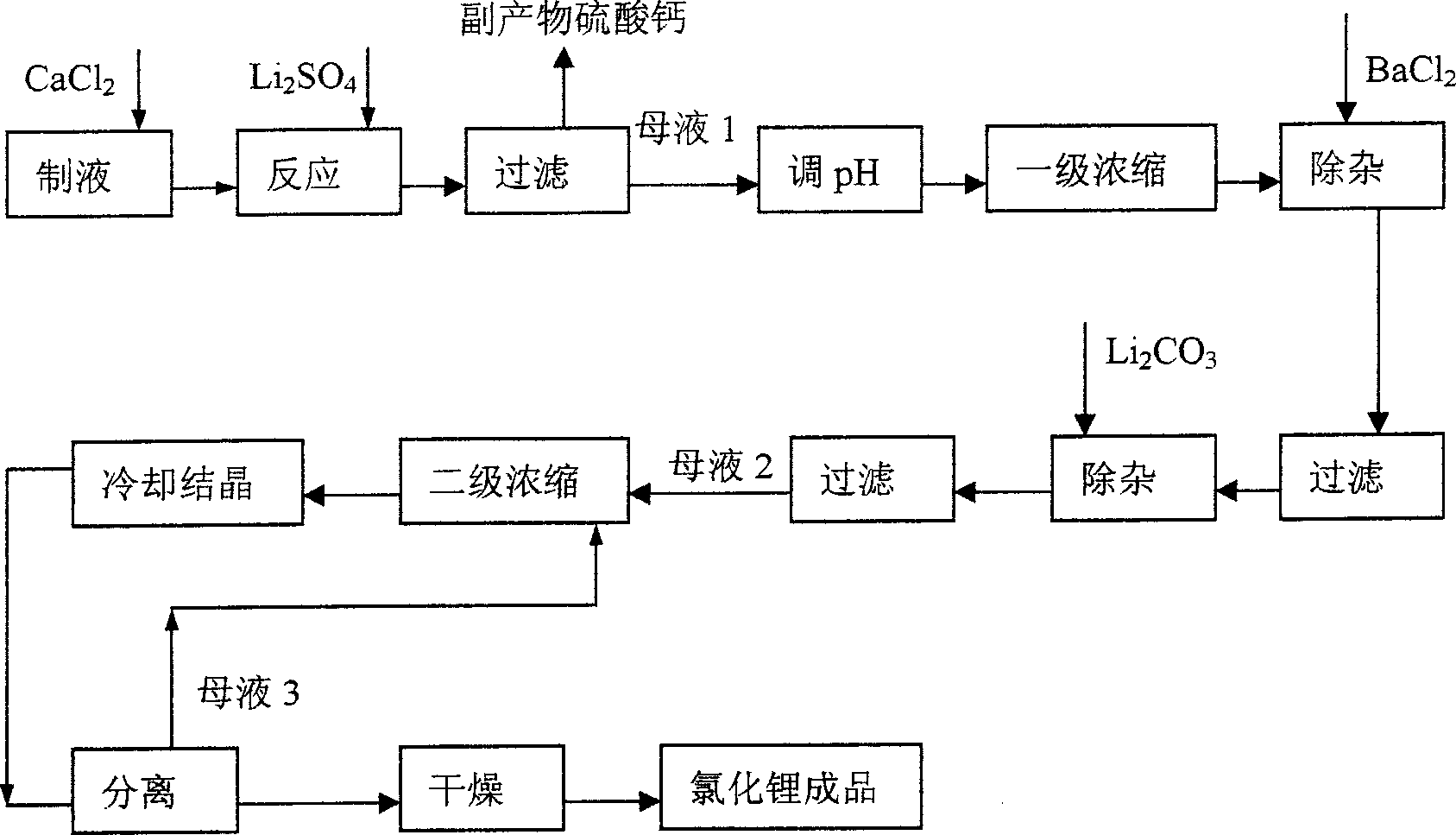
[0033] (1) Pipette concentration is 1.6103mol / L lithium ore leaching liquid-lithium sulfate solution 800ml in the beaker of 2000ml. Place the beaker in a constant temperature water bath at 70°C, slowly add 860 ml of calcium chloride solution with a concentration of 1.5 mol / L while stirring, fully react for half an hour, then keep the temperature and let stand for 1.5 hours.
[0034] (2) vacuum filter the product obtained in step (1), and wash the calcium sulfate filter cake twice with 80° C. deionized water to obtain LiCl solution (mother liquor 1).
[0035] (3) Use hydrochloric acid to adjust the pH value of mother liquor 1 to 7, then concentrate it under reduced pressure to 500ml, measure its SO 4 2- and Ca 2+ content.
[0036] (4) Add 85ml of barium chloride solution of 0.5mol / L concentration while stirring in the concentrated solution of step (3) gained, stir for 20 minutes and filter after leaving standstill for 1 hour, and wash twice with 10 ℃ of deionized water.
[...
Embodiment 2
[0040] The temperature of the constant temperature water bath in step (1) of embodiment 1 is 80°C; the temperature of washing water in step (2) is 85°C, and the rest are the same as in embodiment 1. Analysis and determination of the obtained product LiCl content 99.15%; SO 4 2- Content 0.03%; K+Na 0.3%; other indicators are in line with GB10575-89 industrial first-class standards.
Embodiment 3
[0042] The 1.5mol / L calcium chloride solution that adds in the step (1) of embodiment 1 is 884ml, and the constant temperature water bath temperature is 85 ℃, after reacting for 45 minutes, it is incubated and left standstill for 2 hours; in the step (2), the washing water temperature is 85 ℃ , washing three times; Add the lithium carbonate solution 350ml of 0.1mol / L concentration in the step (4), stir 30 minutes and filter after leaving standstill 2 hours; All the other are with embodiment 1. Analysis and determination of the obtained product LiCl content 99.21%; SO 4 2- The content cannot be detected; K+Na 0.24%; other indicators are in line with the industrial first-class product standard in GB10575-89.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com