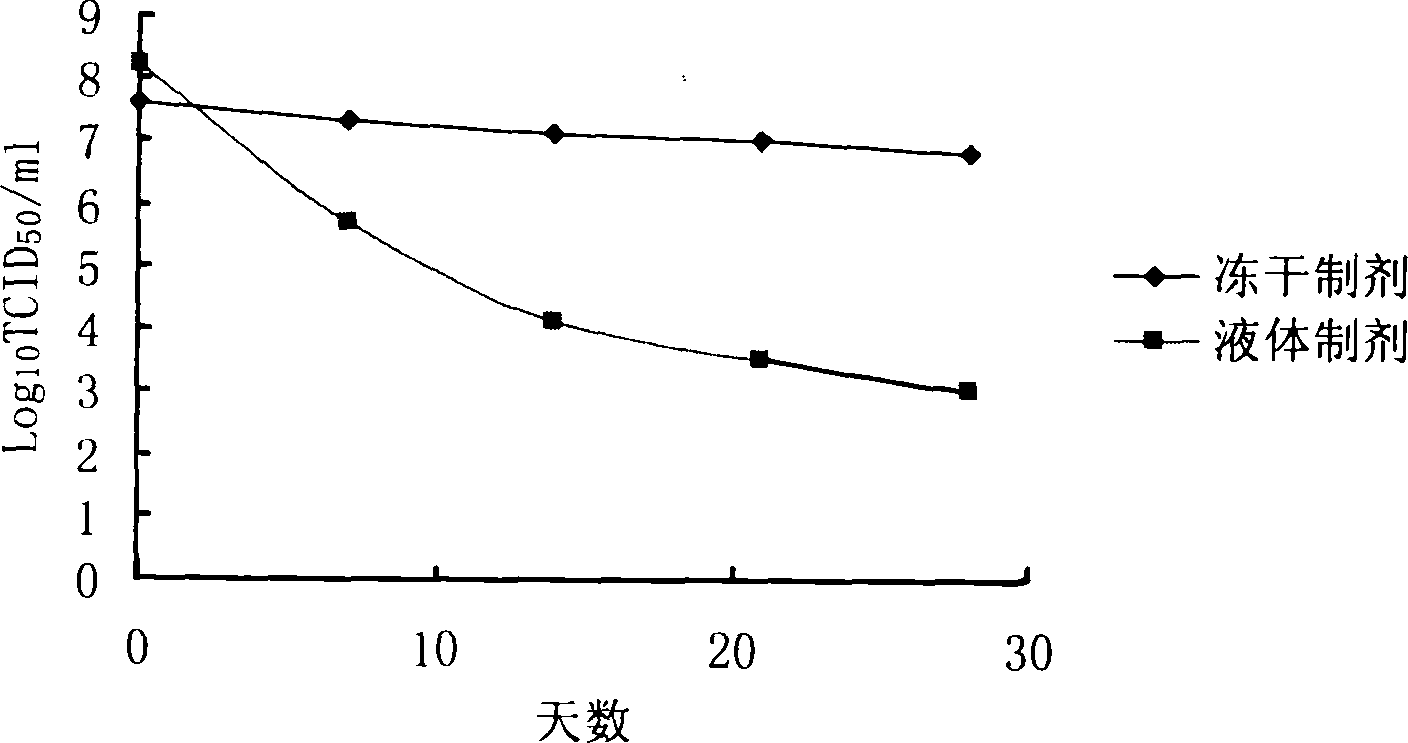
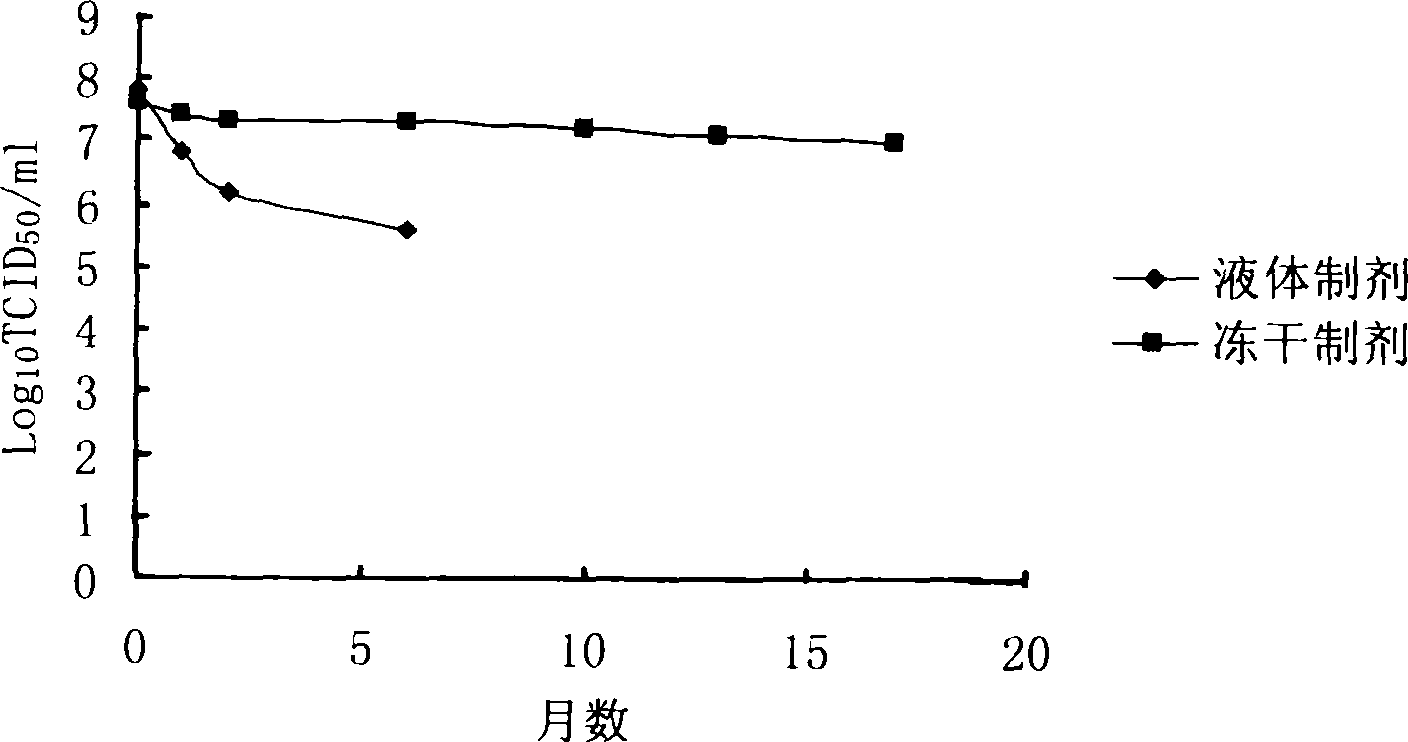
Lyophilized preparation of recombinant adenovirus and preparation method thereof
A technology of recombinant adenovirus and freeze-dried preparation is applied in the field of preparation of freeze-dried dosage form products, can solve the problems such as the lack of freeze-dried adenovirus preparation, and can achieve good application prospect, low cost, good safety and stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation method of the freeze-dried preparation type recombinant adenovirus is: in 100 milliliters of phosphate physiological sodium chloride solution (PBS) with a pH value of 7.0 to 8.0, add 0.5 grams of human serum albumin, 3 grams of trehalose, and 2 grams of mannitol , 2 grams of dextran, 2 grams of sucrose. After fully dissolving, filter and sterilize with a filter membrane with a pore size of 0.22 μm, and then filter the recombinant adenovirus 10 11 TCID 50 The concentrated solution is added to the above-mentioned solution containing the freeze-drying agent for filling, and after each vial is divided into 0.5 milliliters, vacuum freeze-drying starts. The virus liquid was pre-frozen at -35 to -40°C for 3 to 4 hours, and then dried at a shelf temperature of -30 to -32°C and a vacuum of 10 Pa for 8 to 9 hours. After the product is fully formed, the temperature of the shelf is gradually increased from -10°C, 0°C to 32°C, and then dried under a vacuum of 10Pa ...
Embodiment 2
[0026] The preparation method of the freeze-dried preparation type recombinant adenovirus is: in 100 milliliters of phosphate physiological sodium chloride solution (PBS) with a pH value of 7.0 to 8.0, add 0.2 grams of human serum albumin, 2 grams of trehalose, and 1 gram of mannitol. , 1 gram of dextran, 1 gram of sucrose. After fully dissolving, filter and sterilize with a filter membrane with a pore size of 0.22 μm, and then filter the recombinant adenovirus 2×10 12 TCID 50 The concentrated solution is added to the above-mentioned solution containing the freeze-drying agent for filling, and after each vial is divided into 0.5 milliliters, vacuum freeze-drying starts. The virus liquid was pre-frozen at -35 to -40°C for 3 to 4 hours, and then dried at a shelf temperature of -30 to -32°C and a vacuum of 10 Pa for 8 to 9 hours. After the product is fully formed, the temperature of the shelf is gradually increased from -10°C, 0°C to 32°C, and then dried under a vacuum of 10Pa ...
Embodiment 3
[0029] The preparation method of the freeze-dried preparation type recombinant adenovirus is: in 100 milliliters of phosphate physiological sodium chloride solution (PBS) with a pH value of 7.0 to 8.0, add 0.2 grams of human serum albumin, 4 grams of trehalose, and 3 grams of mannitol. , 2 grams of dextran, 3 grams of sucrose. After fully dissolving, filter and sterilize with a filter membrane with a pore size of 0.22 μm, and then filter the recombinant adenovirus 2×10 13 TCID 50 The concentrated solution is added to the above-mentioned solution containing the freeze-drying agent for filling, and after each vial is divided into 0.5 milliliters, vacuum freeze-drying starts. The virus liquid was pre-frozen at -35 to -40°C for 3 to 4 hours, and then dried at a shelf temperature of -30 to -32°C and a vacuum of 10 Pa for 8 to 9 hours. After the product is fully formed, the temperature of the shelf is gradually increased from -10°C, 0°C to 32°C, and then dried under a vacuum of 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com