Chimeric type 5/type 11 or type 35 adenovirus vector for preventing infection with antihuman immunodeficiency virus
An immunodeficiency virus and virus vector technology, which is applied to chimeric type 5/11 or type 35 adenovirus vectors and their medical application fields, can solve problems such as hepatotoxicity, and achieve improved affinity, reduced toxicity, Effectiveness in the treatment and prevention of HIV infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
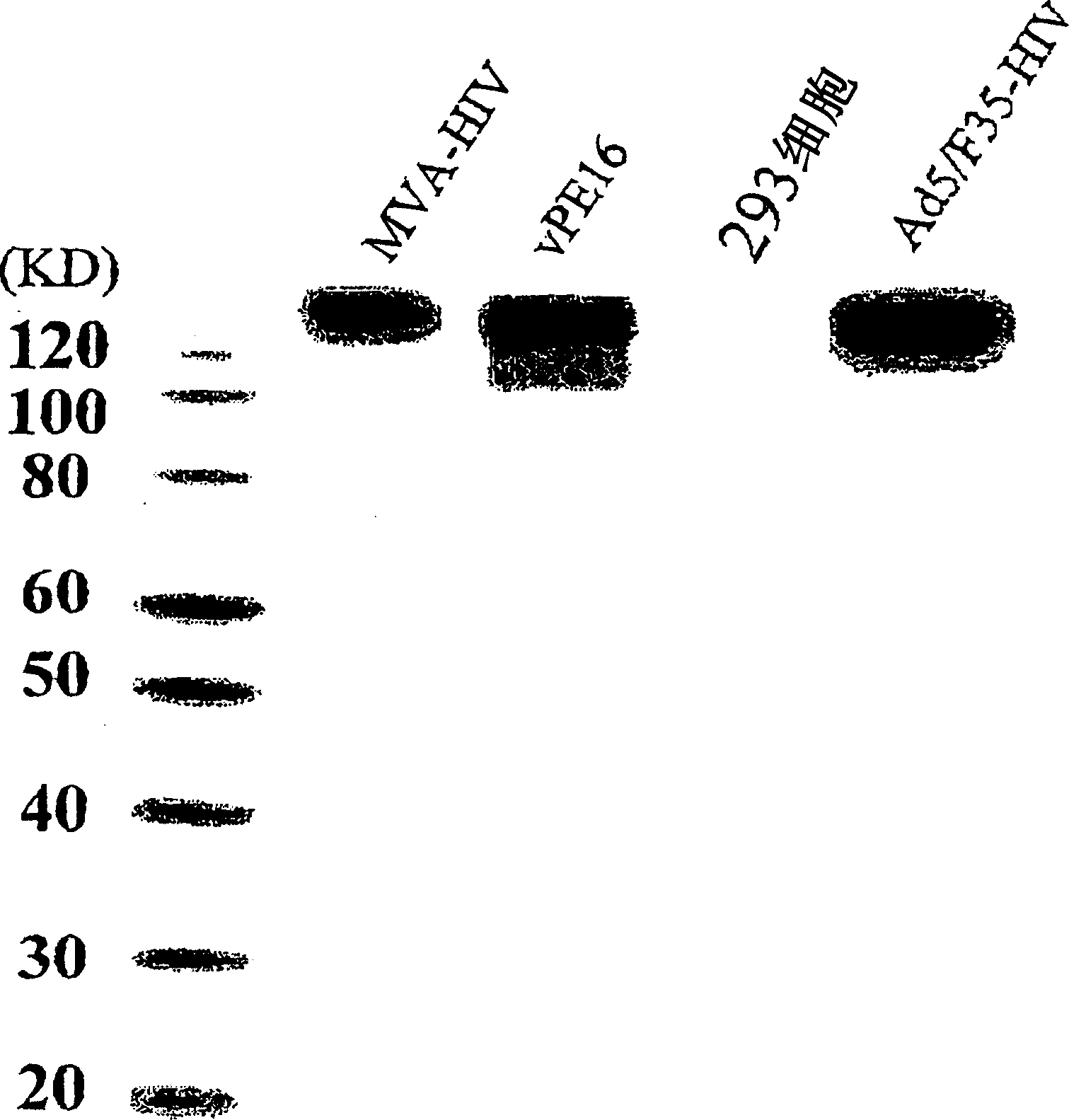
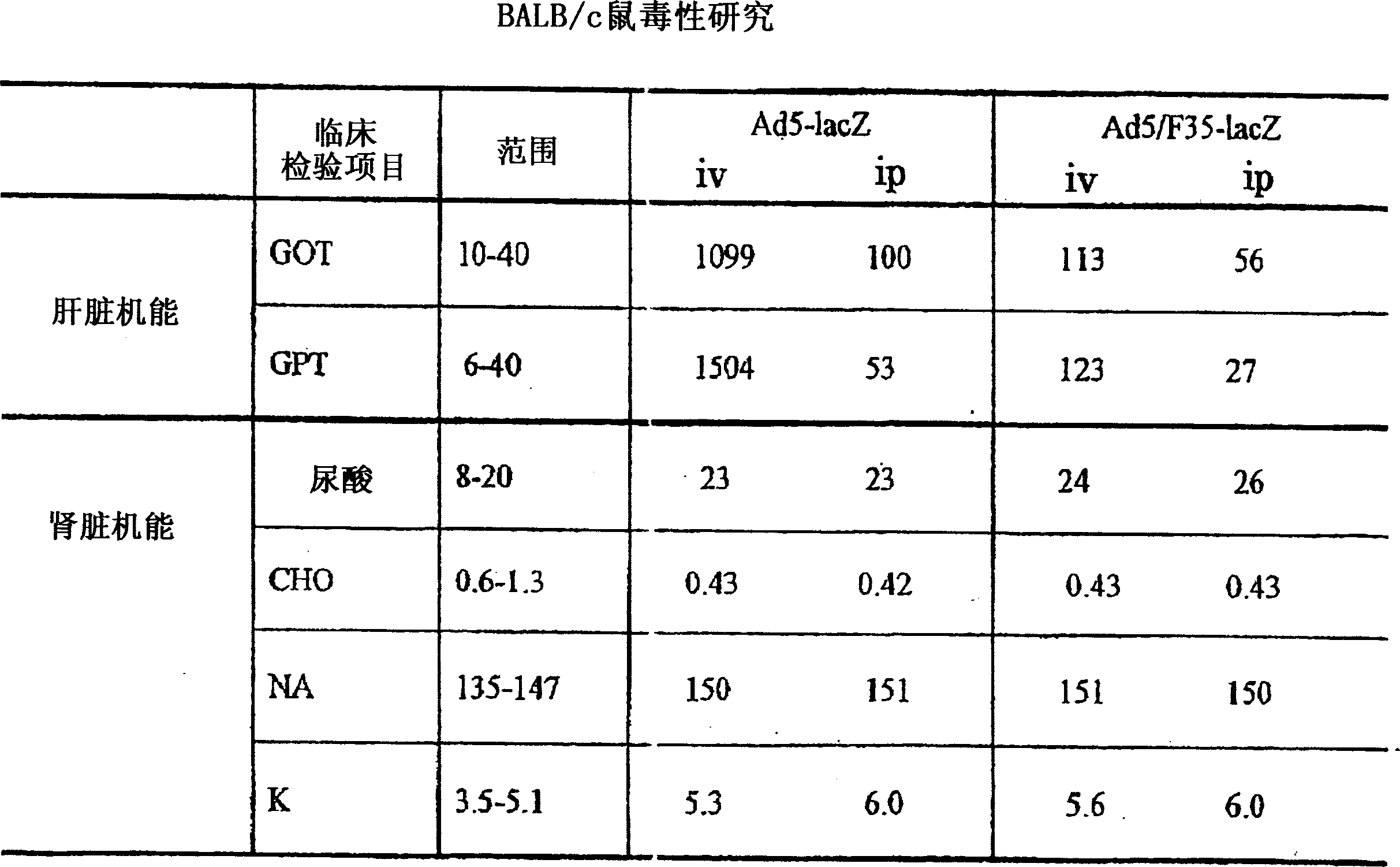
Image
Examples
Embodiment 1
[0078] (1) Construction of chimeric type 5 / type 35 adenovirus vector of the present invention
[0079] Construct the chimeric type 5 / type 35 adenoviral vector of the present invention by using the kit of Avior Therapeutics, Inc (Seattle, WA) as described below, wherein the rev gene and env gene of HIVIIIB of HIVB subtype are expressible The mode is integrated into a replication-deficient type 5 adenovirus with deletion of E1 and E3, the fiber protein coding gene of the type 5 adenovirus is replaced by the fiber protein coding gene of the type 35 adenovirus.
[0080] The construction kit purchased from Avior Therapeutics, Inc (Seattle, WA) includes a left-handed shuttle plasmid (pLHSP) comprising Ad5 22-342 and 3523-5790, E. coli replication origin and amoxicillin resistance gene and A right-handed chimeric shuttle plasmid (pRHSP5 / 35) of a replication-defective adenovirus type 5 comprising deletions of E1 and E3, wherein the fibrin-encoding gene of the adenovirus type 5 is enco...
Embodiment 2
[0094] Evaluation of Recombinant Viruses
[0095] (1) method
[0096] 1) Experimental animals and immunity
[0097] Eight-week-old female BALB / c mice were purchased from Japan's SLC Inc., Japan's Shizuoka, and kept at the Animal Convenience Center on a 12-hour day and night basis. Immunization method reference figure 2 . At 0, 1, and 2 weeks, mice were injected intramuscularly with 100 μg of pCAG dissolved in phosphate buffered saline (PBS) rev / env or pCAG empty plasmid (rev / env removed plasmid) DNA to immunize mice and inject 10 10 Ad5 / F35-LacZ of vp or, intramuscularly or intradermally injected 10 10 vp Ad5 / F35-HIV for booster immunization. For the group immunized with viral vector alone, 10 in PBS 10 Ad5 / F35-LacZ or Ad5 / F35-HIV of vp was administered by intramuscular, intraperitoneal, subcutaneous or intradermal injection.
[0098] 2) Tetramer experiment
[0099] Tetramer assays were performed 1 week after the final immunization. PE-conjugated H-2Dd / p18 tetramer ...
Embodiment 3
[0118] (1) Construction of chimeric type 5 / type 35 adenovirus vector comprising HIV C subtype Env gene / Gag gene
[0119] The chimeric type 5 / type 35 adenovirus vector of the present invention is constructed by the following homologous recombination method, wherein the env gene (gp120, 2kbp) and gag gene (1.5kbp) of HIV C subtype (96ZM651.8 strain) ) expressably integrated into a replication-defective type 5 adenovirus deleted for E1 and E3, the fiber protein-encoding gene of type 5 adenovirus being replaced by the fiber protein-encoding gene of type 35 adenovirus.
[0120] The complete HIV subtype C gp120 coding gene (2kbp, C / gp120) and gag coding gene (1.5kbp, C / gag) (GeneBank No.AF286224) were amplified in recombinant vaccinia virus vT331 strain (Gao F. et al ., J. Virol., 1998, Vol.72, 5680-5690). DNA fragments of C / gp120 and C / gag were synthesized by PCR using the following primers:
[0121] C / gp120-5' Primer: aagaattcctcgagaaaatgagagtgagggagatact
[0122] C / gp120-3' Pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com