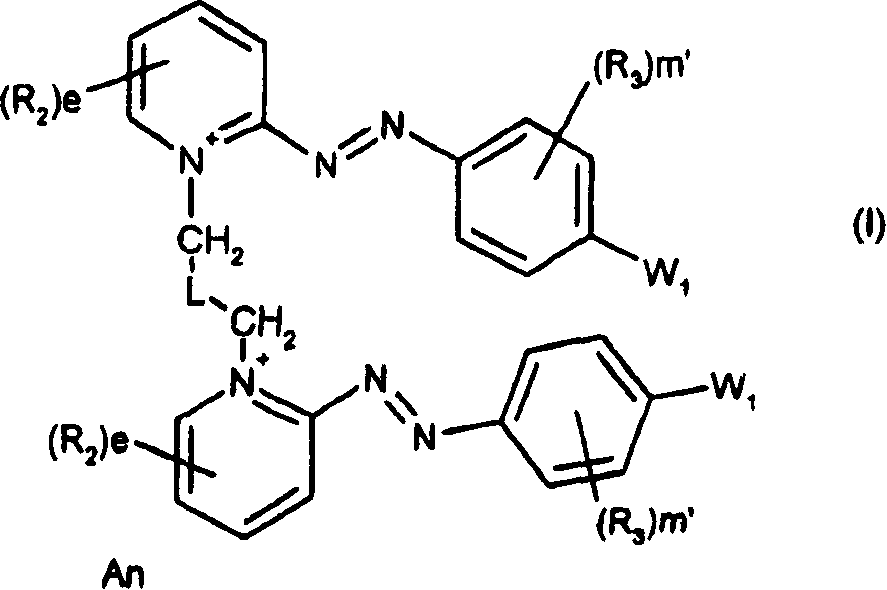
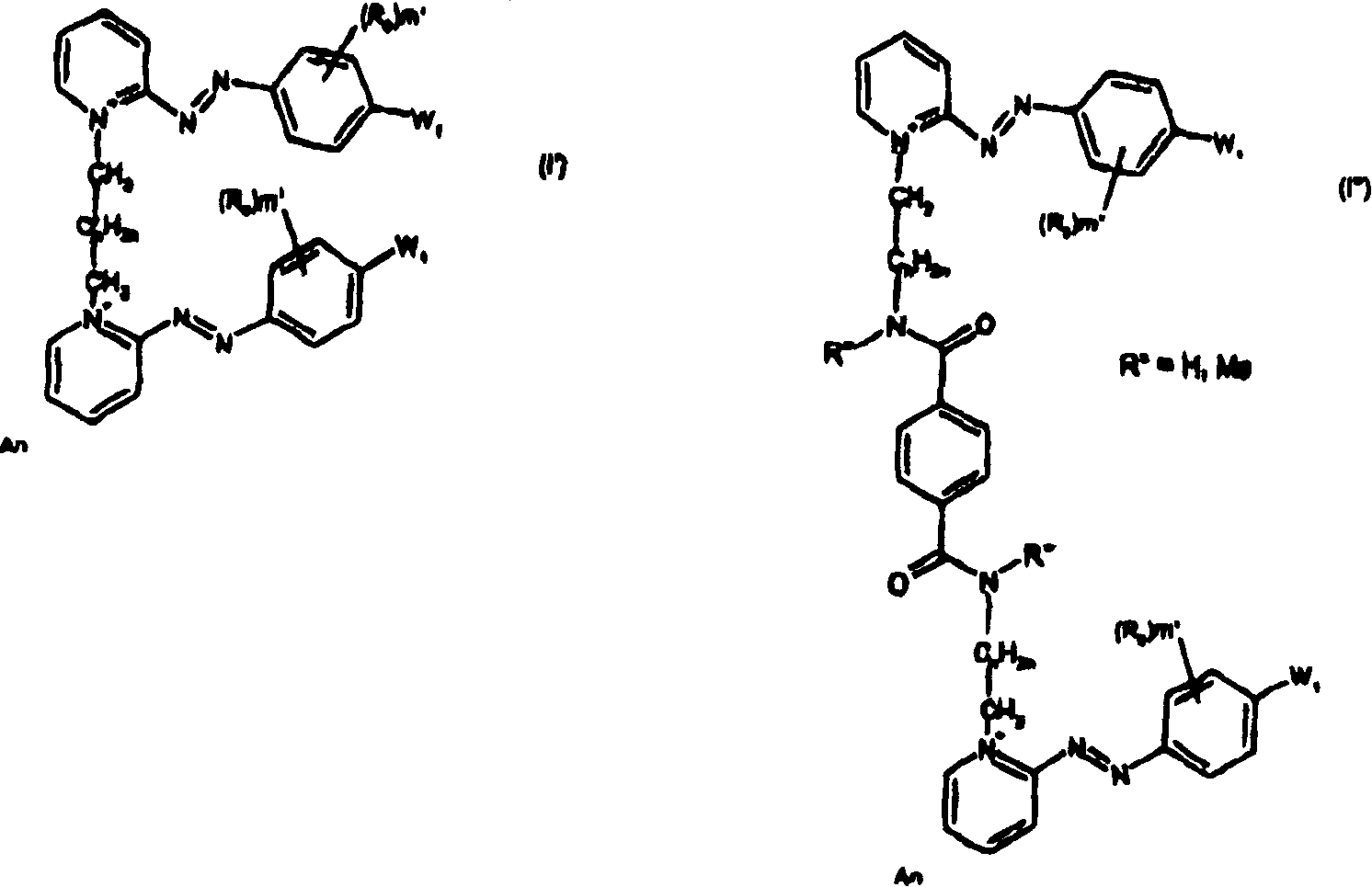
Symmetrical diazo compounds, compositions comprising them, method of coloring, and device
A diazo compound, symmetrical technology, applied in chemical instruments and methods, azo dyes, organic dyes, etc., can solve the problem that the dyeing effect needs to be improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0429] Synthesis of compound 2:
[0430]
[0431] In the presence of 8 ml of 1,6-dibromohexane, compound 1 (Interchim commercial compound, 22.6 g) was reacted in 200 ml of dimethylformamide at 110° C. for 48 hours. It was then allowed to return to ambient temperature and the reaction mixture was poured into 500 ml of diisopropyl ether. The resulting precipitate was filtered off and dried under vacuum. A purple powder corresponding to the compound of structure 2 was obtained.
[0432] 1H NMR and mass spectrometry were consistent with the expected product.
[0433] Synthesis of compound 3:
[0434]
[0435] In the presence of 8 ml of 1,5-dibromopentane, compound 1 (Interchim commercial compound, 22.6 g) was reacted at 110° C. for 4 hours in 150 ml of dimethylformamide. It was then allowed to return to ambient temperature and the reaction mixture was poured into 500 ml of diisopropyl ether. The resulting precipitate was filtered off and dried under vacuum. A purple po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com