Regulation of sperm function
A sperm capacitation and sperm technology, applied in the field of regulation of sperm function, can solve problems such as fertility damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Thawed sperm samples were incubated with supplements as follows:
[0060] 1. Incubate sperm with fibronectin (2 μg / l) for 10 minutes.
[0061] 2. Combine sperm with fibronectin (2 μg / l) plus RGDS (5×10 -6 mol / L) together for 10 minutes.
[0062] 3. Angiotensin II (10 -9 mol / L) was added to fibronectin plus RGDS samples and incubated for an additional 10, 20, 30 and 40 minutes.
[0063] All incubations were performed at 37°C with a water bath and heated stage.
[0064] Semen samples (10 [mu]l) were applied to pre-warmed (37[deg.]C) glass slides, placed on the heated stage of a microscope and examined. The number of motile spermatozoa was counted in the control sample at time zero and in the treated samples after the incubation time.
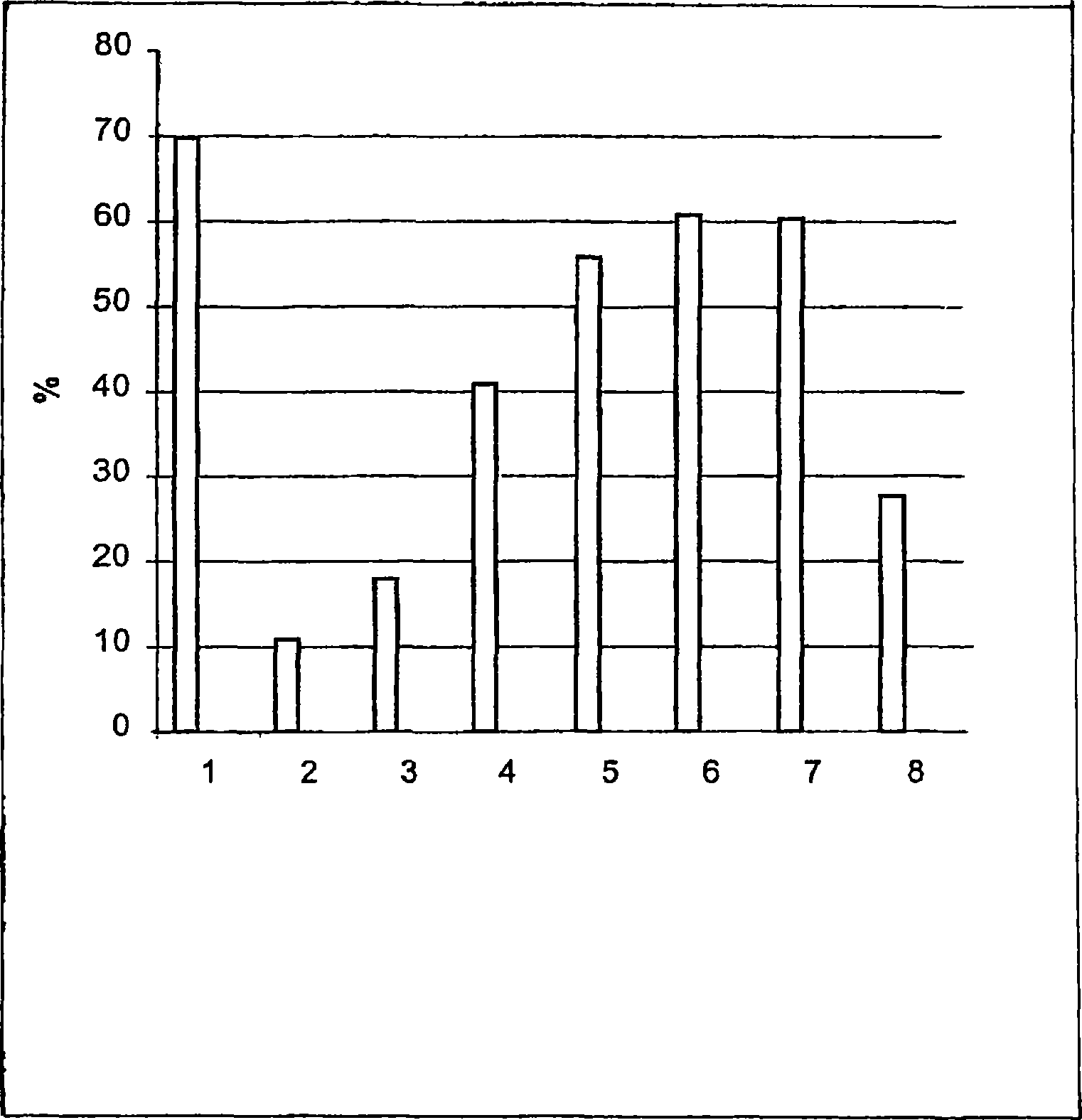
[0065] result in figure 1 is given in , where the bars show the mobility values at the following moments:
[0066] 1. Control 0min;
[0067] 2. Fibronectin for 10 minutes;
[0068] 3. Fibronectin + RGD 10min;
[0069] 4. Fibrone...
Embodiment 2
[0081] Thawed sperm samples were incubated with supplements as follows:
[0082] 1. Incubate sperm with fibronectin (2 μg / l) for 10 minutes.
[0083] 2. Angiotensin II (10 -9 mol / L) was added to the fibronectin samples and incubated for an additional 10, 20 and 30 minutes.
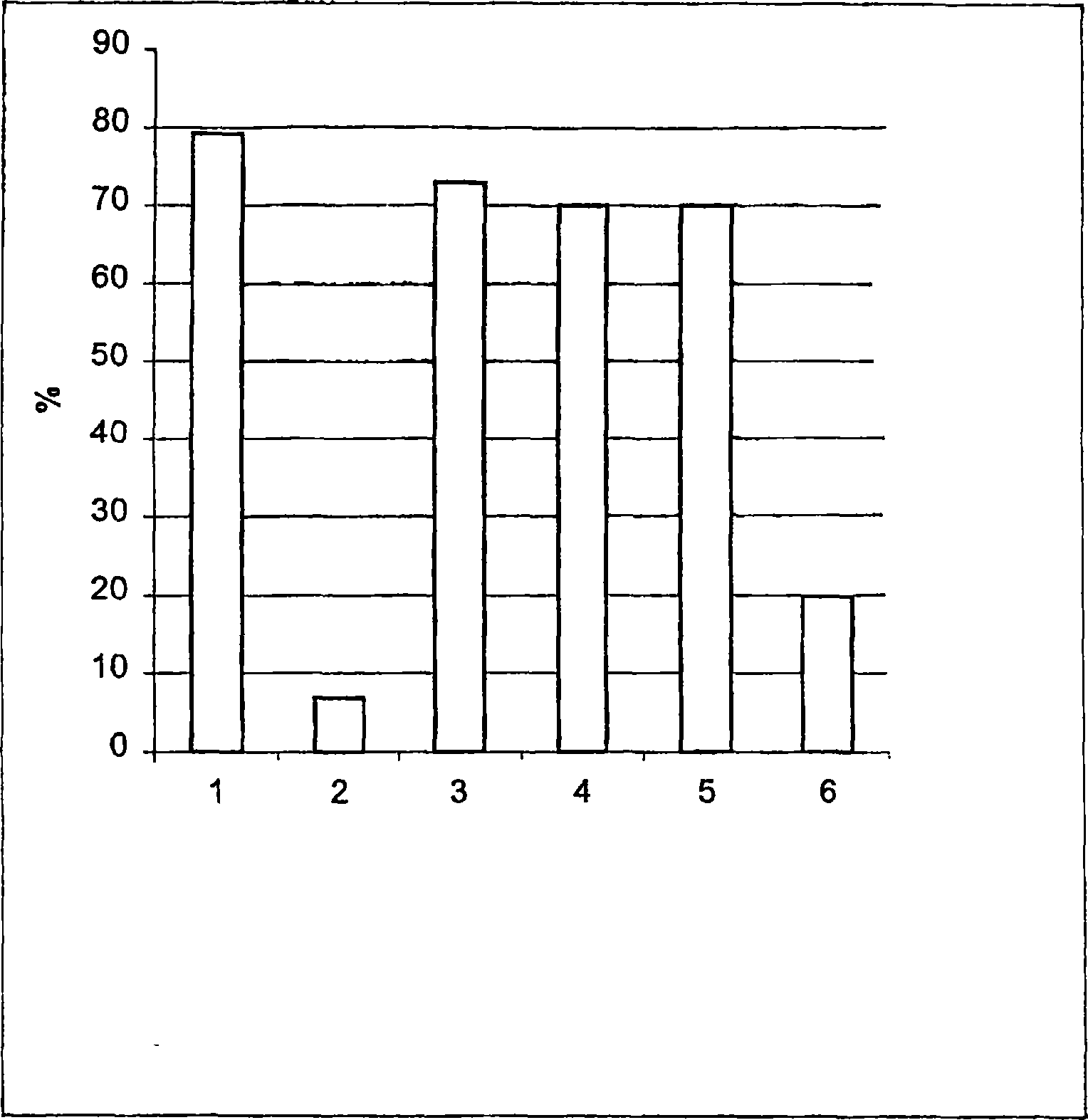
[0084] result in figure 2 is given in , where the bars show the mobility values at the following moments:
[0085] 1) Control 0min;
[0086] 2) Fibronectin for 10 minutes;
[0087] 3) Fibronectin + angiotensin II for 10 minutes;
[0088] 4) Fibronectin + angiotensin II for 20 minutes;
[0089] 5) Fibronectin + angiotensin II for 30 minutes;
[0090] 6) After 30 minutes of control;
[0091] It can be seen from the figure:
[0092] a) The control sample showed 79% motility at time zero;
[0093] b) The effect of fibronectin concentration on spermatozoa shows very significant changes in total sperm motility. Only 8% of the sperm motility was present after 5-10 minutes of incubation.
[0094] b)...
Embodiment 3
[0097] Thawed sperm samples were incubated with supplements as follows:
[0098] 1. Combine the sperm with RGDS (5×10 -6 mol / L) together for 5 minutes.
[0099] 2. Add fibronectin (2 μg / l) to RGD S samples and incubate for another 5 min.
[0100] 3. Angiotensin II (10 -9 mol / L) was added to the fibronectin+RGDS samples and incubated for another 10, 20 and 30 minutes.
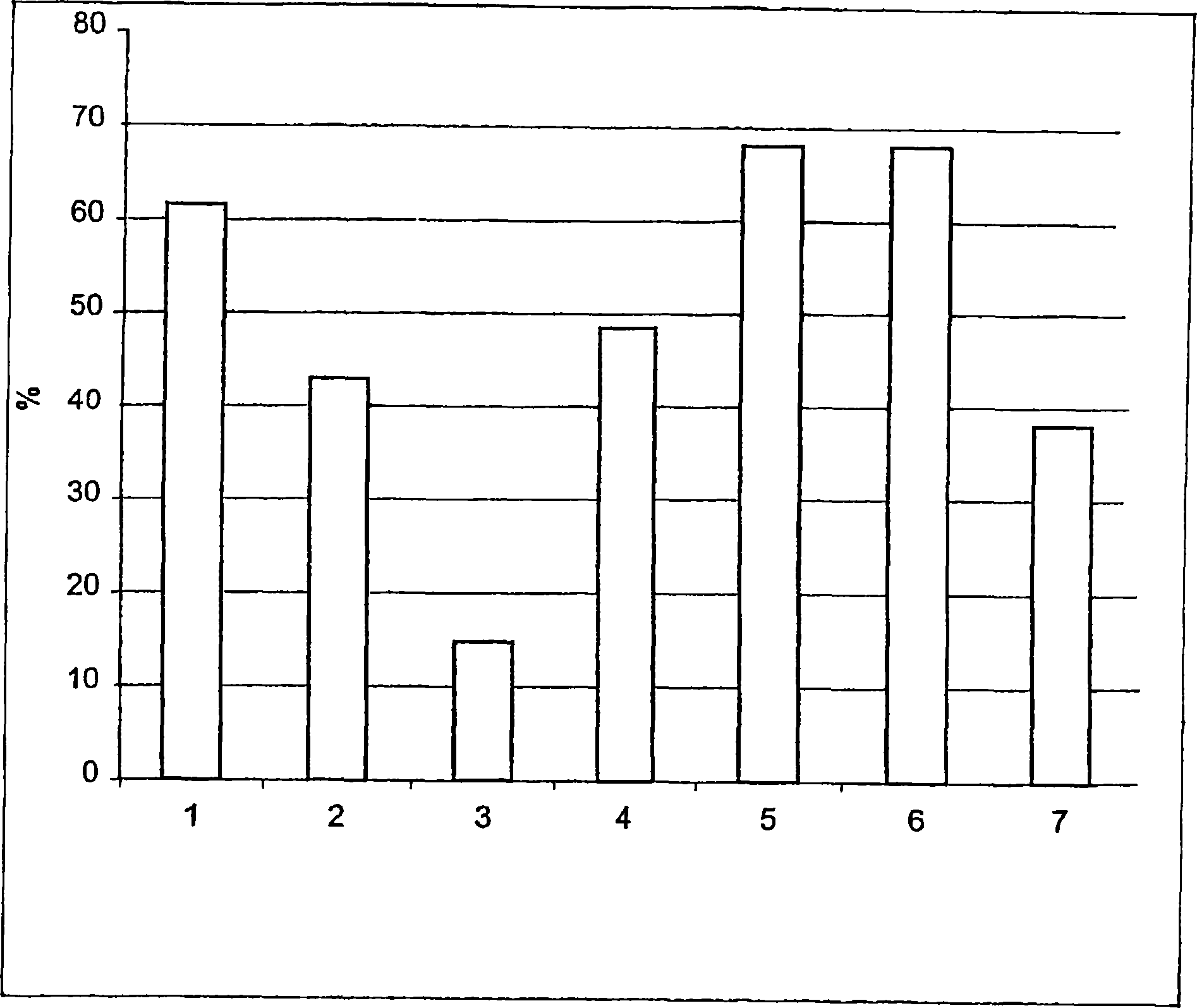
[0101] result in image 3 is given in , where the bars show the mobility values at the following moments:
[0102] 1) Control 0min;
[0103] 2) Pre-incubate with RGD for 5 minutes;
[0104] 3) RGD+fibronectin for 5min;
[0105] 4) Fibronectin + RGD + angiotensin II for 10 minutes;
[0106] 5) Fibronectin + RGD + angiotensin II for 20 minutes;
[0107]6) Fibronectin + RGD + angiotensin II for 30 minutes;
[0108] 7) Control 30min;
[0109] It can be seen from the figure:
[0110] a) The control sample showed 62% motility at time zero;
[0111] b) Pre-incubation of RGDS with sperm showed a 19% reduc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com