A process for the resolution of nefopam
A technology of nefopam and resolution agent, which is applied in the field of preparing single enantiomers of nefopam, can solve problems such as different biological activities, and achieve the effect of easy recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1 Nefopam free base
[0016] Racemic Nefopam hydrochloride (5.0 Kg, 17.2 mol) was suspended in water (12.5 L) and 2M sodium hydroxide solution (18.5 Kg), and solid sodium hydroxide (50 g) was added. Ethyl acetate (11.16 Kg) was added and the mixture was stirred for 10 minutes until complete dissolution was achieved. Stirring was stopped and the two layers separated. The ethyl acetate layer was removed and stored. The aqueous layer was further extracted with ethyl acetate (11.16 Kg) and the combined ethyl acetate extracts were dried over magnesium sulfate (500 g), filtered and evaporated to give the product as a colorless semi-solid. The above procedure was repeated to obtain the product in quantitative yield (9.31 Kg, 106%, with residual ethyl acetate).
Embodiment 2
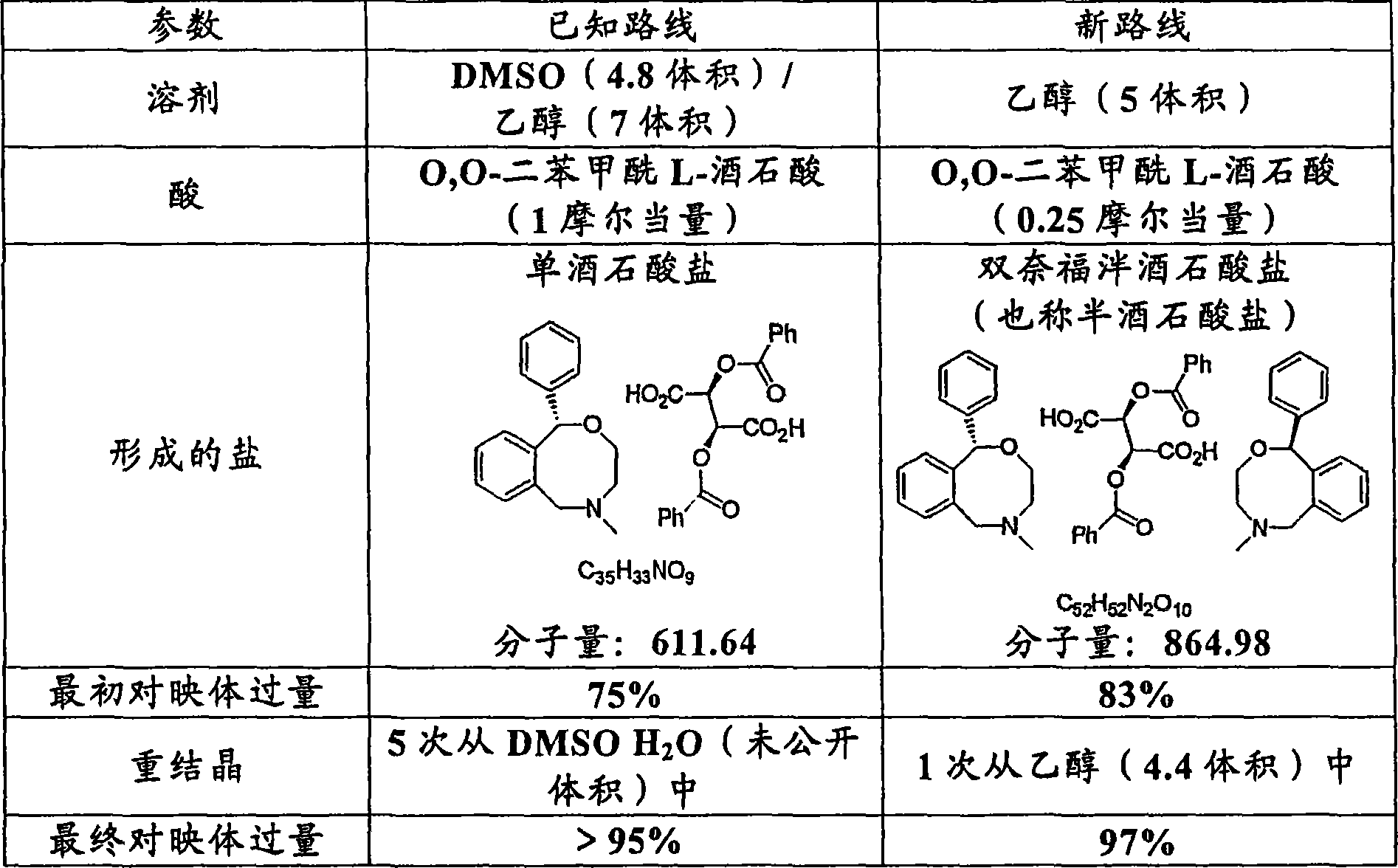
[0017] Example 2 (+)-bis-nefopam O, O-dibenzoyl-l-tartrate
[0018] The isolated product of Example 1 (7.86 Kg, 31.0 mol) was dissolved in ethanol (14.7 Kg) and stirred at room temperature. A solution of O,O-dibenzoyl-L-tartaric acid (2.75 Kg, 0.25 molar equivalents) in ethanol (16.0 Kg) was added over a period of 20 minutes. The resulting solution was allowed to stir overnight at room temperature, during which time crystallization occurred. Crystals were collected by filtration, washed with ethanol (2 x 2 L) and dried at 45°C under reduced pressure to constant weight. The product was obtained as a colorless solid, 4.27 Kg, 32%. Chiral HPLC showed 83% (+)-Nefopam enantiomeric excess.
[0019] The solid was recrystallized in two batches from ethanol (2 x 12.16 Kg) and washed with ethyl acetate (2 x 2 L). The combined solids were dried under reduced pressure at 45°C to constant weight to obtain the product as a colorless solid, 2.90 Kg, 68%. Chiral HPLC analysis showed 99% ...
Embodiment 3
[0021] Embodiment 3 (+)-Nefopam
[0022] Sodium hydroxide (335g, 8.38mol, 2.5eq) was dissolved in water (11.9Kg) and the solution was added to the isolated product of Example 2 (2.89Kg, 3.34mol). The mixture was stirred for 10 minutes and extracted with ethyl acetate (3 x 4.38 Kg). The ethyl acetate extract was dried over magnesium sulfate (500 g), filtered and evaporated to constant weight under reduced pressure. The product was isolated as a colorless oil, 1.53 Kg, 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com