Preparation method of calcium sulfate nanometer material
A nanomaterial, calcium sulfate technology, applied in the field of preparation of calcium sulfate nanomaterials, can solve the problems of high reaction temperature requirements, difficult large-scale production, complex operation, etc., and achieve the effects of reduced production costs, convenient operation, and enhanced fluorescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
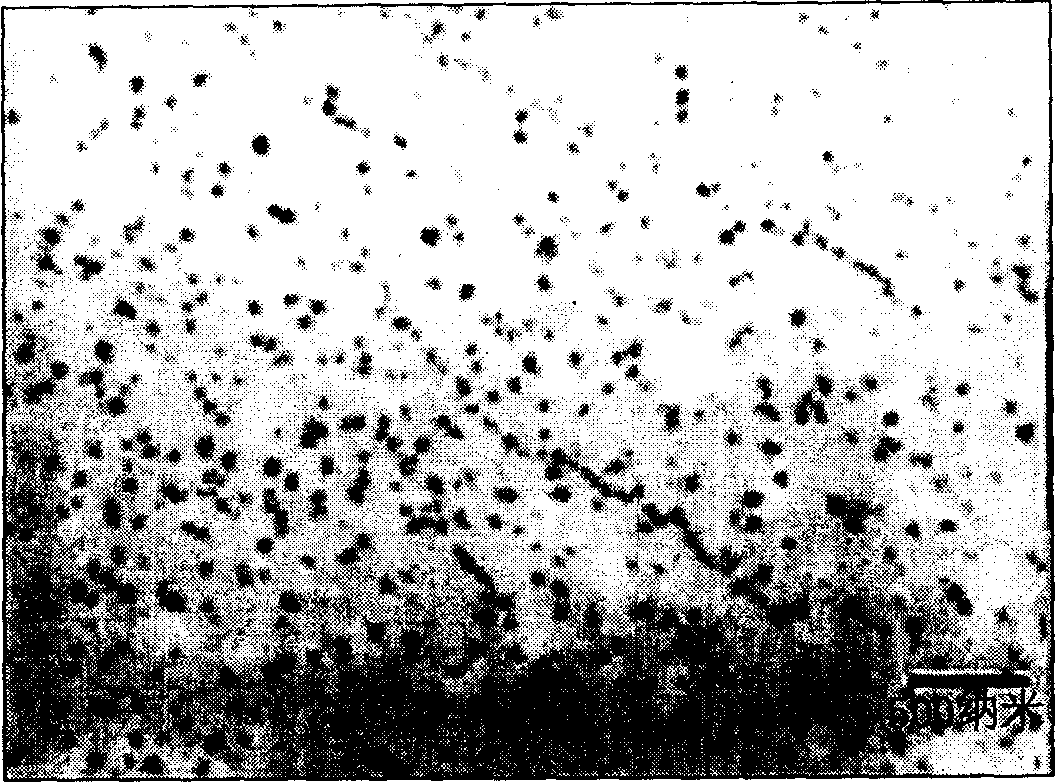
Image
Examples
Embodiment 1
[0037] (1) Weigh 0.0025 moles of CaCl respectively 2 and Na 2 SO 4 , and then were dissolved in 25 ml of distilled water to make CaCl with a molar concentration of 0.10 mol / L 2 and Na 2 SO 4 solution;
[0038] (2) Add 1.5 milliliters of any solution prepared in step (1) to the Erlenmeyer flask in sequence, 5 milliliters of C 12 E. 9 1 milliliter of n-pentanol and 40 milliliters of cyclohexane were stirred for 5 minutes to form a transparent and uniform reversed-phase micelle A solution, and another solution in step (1) was prepared into another reversed-phase micelle B in the same way solution;
[0039] (3) Mix the two reversed-phase micellar solutions obtained in step (2), stir for 2 minutes to make them fully mixed, and after standing for 1 minute, the reversed-phase micellar solution is demulsified and separated with acetone, and the acetone is added The amount is 5 milliliters, the product is washed three times with absolute ethanol and distilled water, and vacuum-...
Embodiment 2
[0042] (1) Weigh 0.0025 moles of CaCl respectively 2 and Na 2 SO 4 , and then were dissolved in 25 ml of water to make CaCl with a molar concentration of 0.10 mol / L 2 and Na 2 SO 4 solution;
[0043] (2) A: Add 1.5 milliliters of CaCl prepared in step (1) to the Erlenmeyer flask2 solution, 5 ml C 12 E. 9 , 1 milliliter of n-pentanol and 40 milliliters of cyclohexane, stirred to form a transparent and uniform reversed-phase micellar solution;
[0044] B: Add 1.5 milliliters of Na prepared in step (1) respectively to the Erlenmeyer flask 2 SO 4 solution, 5 ml C 12 E. 9 , 1 milliliter of n-pentanol and 40 milliliters of cyclohexane, stirred to form another transparent and uniform reversed-phase micellar solution;
[0045] (3) Stir two kinds of reversed-phase micellar solutions of A and B gained in step (2), make it mix uniformly, let it stand for reaction for 5 minutes, separate the reversed-phase micellar solution with acetone demulsification, and the acetone addition...
Embodiment 3
[0047] (1) Weigh 0.0025 moles of CaCl respectively 2 and Na 2 SO 4 , and then were dissolved in 25 ml of water to make CaCl with a molar concentration of 0.10 mol / L 2 and Na 2 SO 4 solution;
[0048] (2) A: Add 1.5 milliliters of CaCl prepared in step (1) to the Erlenmeyer flask 2 solution, 5 ml C 12 E. 9 , 1 milliliter of n-pentanol and 40 milliliters of cyclohexane, stirred to form a transparent and uniform reversed-phase micellar solution;
[0049] B: Add 1.5 milliliters of Na prepared in step (1) respectively to the Erlenmeyer flask 2 SO 4 solution, 5 ml C 12 E. 9 , 1 milliliter of n-pentanol and 40 milliliters of cyclohexane, stirred to form another transparent and uniform reversed-phase micellar solution;
[0050] (3) Stir two kinds of reversed-phase micellar solutions of A and B obtained in step (2), make it mix uniformly, leave to stand for reaction for 12 hours, separate the reversed-phase micellar solution with acetone demulsification, the acetone additio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com