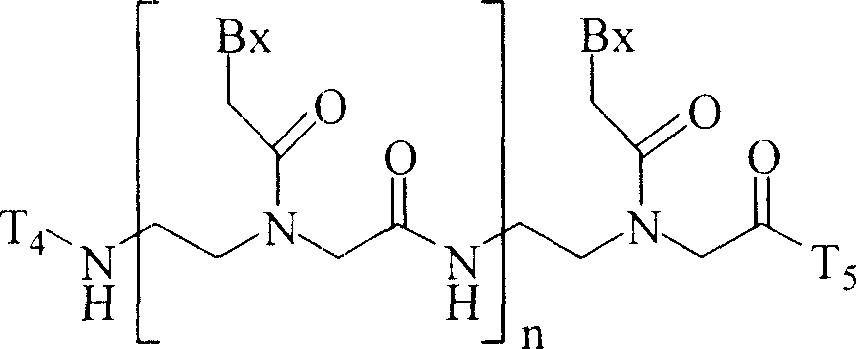
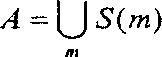Antisense oligonucleotide modulation of STAT3 expression
An oligonucleotide and nucleic acid molecule technology, applied in the field of compositions for regulating the expression of human STAT3 gene, and DNA-binding proteins, which can solve the problems of incomplete selection of patients with multiple myeloma, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0082] 教授上面含磷键的制备的代表性美国专利包括但不限于美国专利:3,687,808;4,469,863;4,476,301;5,023,243;5,177,196;5,188,897;5,264,423;5,276,019;5,278,302;5,286,717;5,321,131;5,399,676;5,405,939;5,453,496;5,455,233;5,466,677 ;5,476,925;5,519,126;5,536,821;5,541,306;5,550,111;5,563,253;5,571,799;5,587,361;5,194,599;5,565,555;5,527,899;5,721,218;5,672,697和5,625,050,每项专利在文中引用作为参考。
[0083] In a more preferred embodiment of the invention, the oligomeric compound has one or more phosphorothioate linkages and / or heteroatom internucleoside linkages, in particular -CH 2 -NH-O-CH 2 -, -CH 2 -N(CH 3 )-O-CH 2 -[called methylene (methylimino) or MMI backbone], -CH 2 -O-N(CH 3 )-CH 2 -, -CH 2 -N(CH 3 )-N(CH 3 )-CH 2 -and-O-N(CH 3 )-CH 2 -CH 2 -[wherein the natural internucleotide phosphodiester bond is represented as -O-P(=O)(OH)-O-CH 2 -]. MMI-type internucleoside linkages are disclosed in US Patent 5,489,677, cited above. Preferred internucleotide amide linkages are disclosed in US Patent 5,602,240, ...
Embodiment 1
[0324] Example 1: Synthesis of oligonucleotides
[0325] Unmodified oligodeoxynucleotides were synthesized on an automated DNA synthesizer (Applied Biosystems, model 380B) using standard phosphoramidite chemistry via iodine oxidation. β-cyanoethyldiisopropyl-phosphoramidite was purchased from Applied Biosystems (Foster City, CA). For phosphorothioate oligonucleotides, the standard oxidation bottle was replaced with 0.2M in acetonitrile 3 H-1,2-Benzenedithiol-3-one 1,1-dioxide solution for stepwise thialation of phosphite bonds. The thiamination cycle wait step was increased to 68 seconds and followed by a capping step. The cytosine may be 5-methylcytosine (5-methyldeoxycytidine phosphoramidite is available from Glen Research, Sterling, VA or Amersham Pharmacia Biotech, Piscataway, NJ).
[0326] 2'-Methoxy β-cyanoethyldiisopropyl-phosphoramidite (Chemgenes, Needham, MA) and the standard cycle for unmodified oligonucleotides (except for the following pulse delivery of tetrazo...
Embodiment 2
[0375] Embodiment 2: human STAT3 oligodeoxynucleotide sequence
[0376]Antisense oligonucleotides targeting human STAT3 were designed. The target sequence data are from the APRF cDNA sequence published by Akira, S. et al. (Cell, 1994, 77, 63-71); GenBank _ Accession number L29277, herein SEQ ID NO:1. A series of oligodeoxynucleotides with phosphorothioate linkages were synthesized. 2'-Deoxycytosine is 5-methylcytosine. These oligonucleotide sequences are shown in Table 1. Another set of oligonucleotides was synthesized as chimeric oligonucleotides ("gapmers"), 20 nucleotides in length consisting of a central "gap" region containing 10 2'-deoxynucleotides and It consists of 5-nucleotide "wings" on both sides (5' and 3' directions). The wings consist of 2'-O-methoxyethyl (2'-MOE) nucleotides. The internucleoside (backbone) linkages are phosphorothioate linkages (P=S) throughout the oligonucleotide. All 2'-MOE cytosines and 2'-deoxycytosines are 5-methylcytosines. These o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com