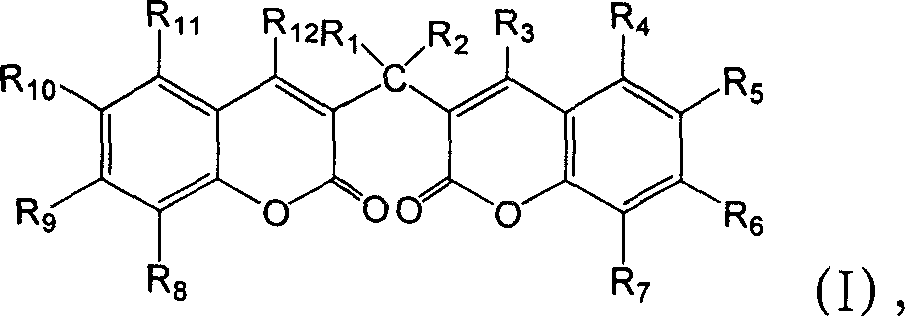
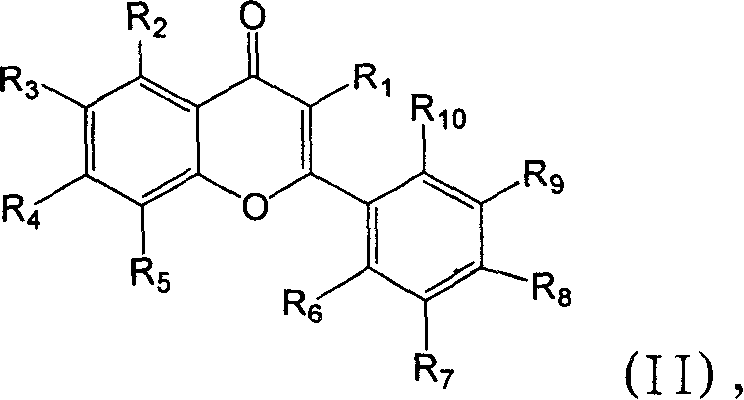
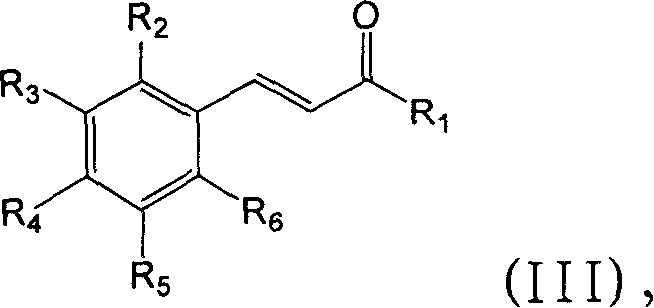
Modulation of peroxisome proliferation-activated receptors
A technology of compounds and modulators, applied in the field of DNA vectors, can solve the problems of limited therapeutic efficacy and side effects of PPAR
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
[0163] Example 3 PGR3 / ZADH2 Small Molecule Inhibitors
[0164]The recombinant human PGR3 / ZADH2 protein was expressed and its enzyme activity was detected. Similar to murine PGR3 / ZADH2, recombinant human PGR3 / ZADH2 has 15-ketoprostaglandin-Δ 13 - Reductase activity and catalyzes the conversion of 15-ketoprostaglandins to 13,14-dihydro-15-ketoprostaglandins.
[0165] The inhibitory effect of compounds 1-49 on PGR3 / ZADH2 activity was tested. These compounds are available commercially (eg, from Sigma-Aldrich, St. Louis, MO) or can be prepared from methods well known in the art. Inhibition assays were performed as described above. Different concentrations of PGR3 / ZADH2 inhibitors were added to the reaction mixture and incubated at 37°C for 2 hours.
[0166] More specifically, 50 μM of compounds 8, 12, 13, and 27, and 25 μM of trifluorobenzyl-2-hydroxycinnamic acid (compound 22) were added to the PGR3 / ZADH2 enzyme reaction system, and the enzymatic reduction of 15-ketoprostatic ...
Embodiment 4
[0170] Effect of Example 4 PGR3 / ZADH2 Inhibitor on Insulin Sensitivity
[0171] The effect of two of the aforementioned PGR3 / ZADH2 inhibitors, compounds 12 and 28, on insulin sensitivity was examined as follows. 3T3-L1 cells were induced to differentiate in the same manner as above. According to the method described by Finga et al. (Endocrinology 134:728-735), by measuring the response to 2-deoxy-D-[ 3 H] Glucose uptake was performed in a glucose transport assay. 1.67M inhibitor or 45nMPPAR-γ agonist, Avandia, was added to the cells.
[0172] 10M Cytochalasin B was used to measure background levels of glucose uptake. 100 nM insulin was used to stimulate glucose uptake. Insulin treatment alone increases glucose uptake by more than 10-fold in differentiated 3T3-L1 cells. Glucose uptake was increased 30-fold in the presence of any two PGR3 / ZADH2 inhibitors. The ability of these inhibitors to increase glucose uptake was found to be comparable to that of AVANDIA.
[0173] Th...
Embodiment 5
[0176] Example 5 RNA interference
[0177] Silencing of PGR3 / ZADH2 expression by RNA interference in 3T3-L1 preadipocytes. To generate vectors encoding interfering RNAs targeting PGR3 / ZADH2, standard methods were used to synthesize vectors containing 5'-GAT CCG TGC CAC GTT ATC GGA ACC TTC AAG AGA GGT TCC GAT AAC GTGGCA CTT TTT TGG AAA-3' (SEQ ID NO: 9; forward primer) and oligonucleotides of AGC TTT TCC AAA AAAGTG CCA CGT TAT CGG AAC CTC TCT TGA AGG TTC CGA TAA CGT GGC ACG-3' (SEQ ID NO: 10; reverse primer). They were then annealed and introduced into the linear siRNA expression vector, pSilencer™ neo (catalog # 5764, Ambion). The siRNA expression vector was then introduced into 3T3-L1 preadipocytes by electroporation. Stable transfected clones were isolated using G418 selection. PGR3 / ZADH2 mRNA levels in these claims were then detected using standard RT-PCR. It was found that PGR3 / ZADH2 mRNA transcript levels were suppressed.
[0178] The clones described above were used...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com