Process of separating endothelial ancestral cell from human fat tissue
An endothelial progenitor cell and separation method technology is applied in the field of obtaining seed cells, and achieves the effects of low cost, wide source of raw materials and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Method for isolating endothelial progenitor cells from human adipose tissue
[0043] (1) Human adipose tissue was digested with 0.01% type II collagenase for 30 minutes;
[0044] (2) Centrifuge at 1200g for 5 minutes;
[0045] (3) with 5×10 4 piece / cm 2 Inoculate on a fibronectin (FN)-coated culture dish, cultivate with DMEM medium containing 2% fetal bovine serum, and change medium for 6 hours;
[0046] (4) When the cells are fused to 80-90%, subculture, the subculture density is 2×10 4 piece / cm 2 , after in vitro amplification to the second generation, induction is carried out.
Embodiment 2
[0048] cell induction
[0049] The second-generation cells obtained by the method described in Example 1 were divided into induction group and non-induction group, and the non-induction group was cultured with DMEM medium containing 2% fetal bovine serum; the induction group was cultured with 2% fetal bovine serum and VEGF, Culture in EGM-2 medium with growth factors such as bFGF.
Embodiment 3
[0051] detection
[0052] 1. Observation of cell morphology
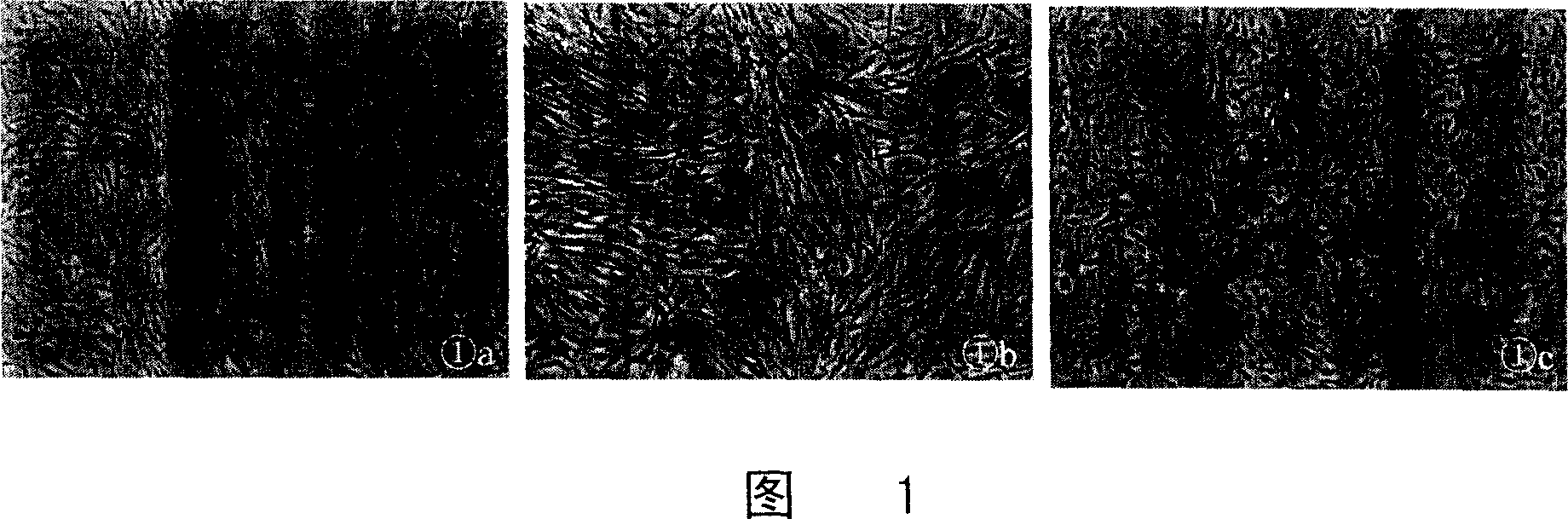
[0053] The results are shown in Figure 1. After 12 days of induction, the cells showed a typical endothelial cell "paving stone" structure. The primary cells were spindle-shaped cells (Figure 1a); the second-generation cells in the non-induced group were still spindle-shaped (Figure 1b), while the cells in the induced group showed a typical "paving stone"-like arrangement (Figure 1c).
[0054]Normal endothelial cells have a "paving stone" like morphology. We transformed the received "spindle-shaped" cells into an endothelial-like morphology under appropriate culture conditions. Morphologically, the "spindle" cells transformed into a normal endothelial cell-like morphology, suggesting that the isolated "spindle" cells are precursors (ie, progenitors) of endothelial cells.
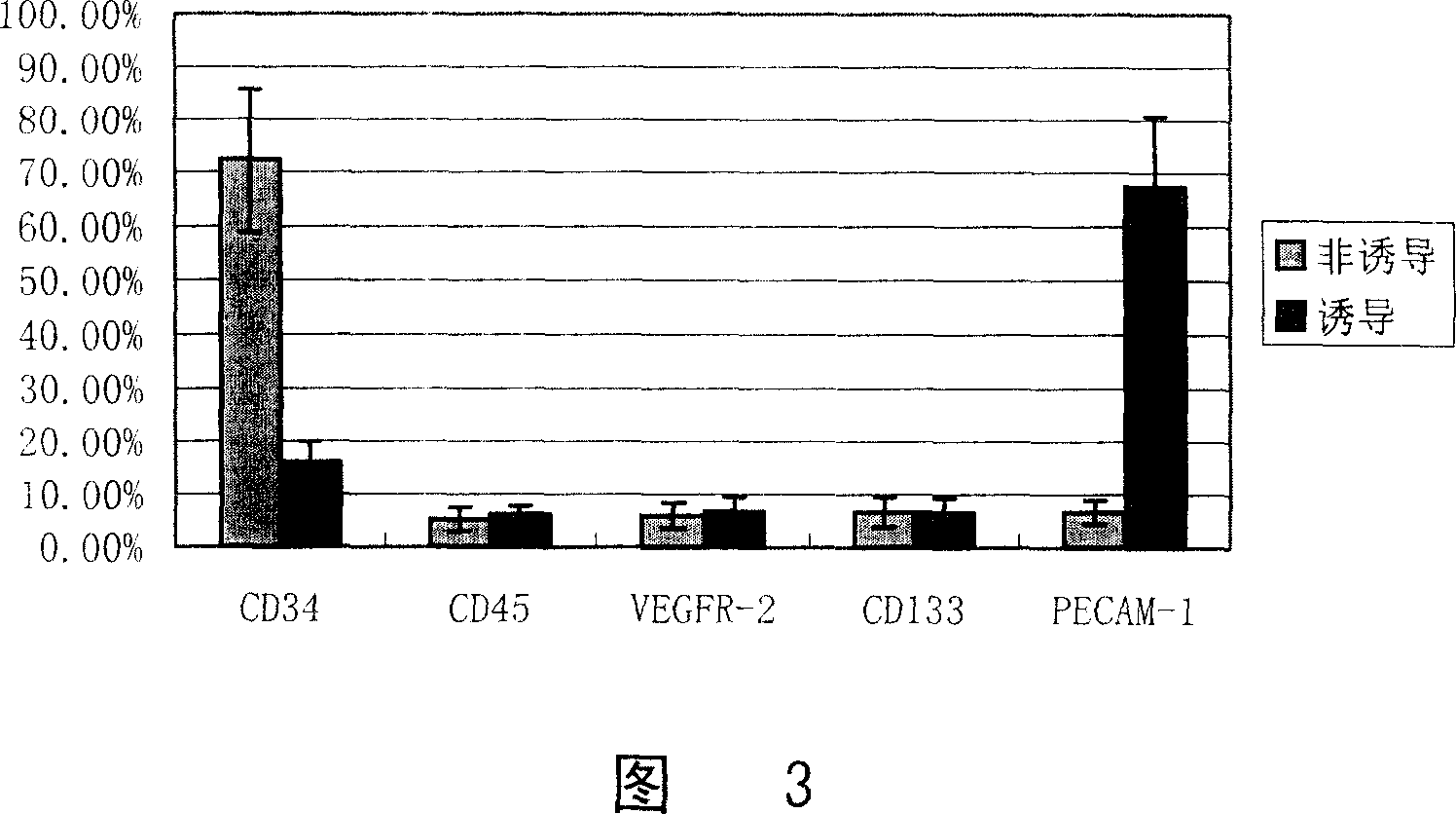
[0055] 2. Cell immunofluorescence detection:
[0056] After culturing and induction for 12 days respectively, fix the cells with ethanol:acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com