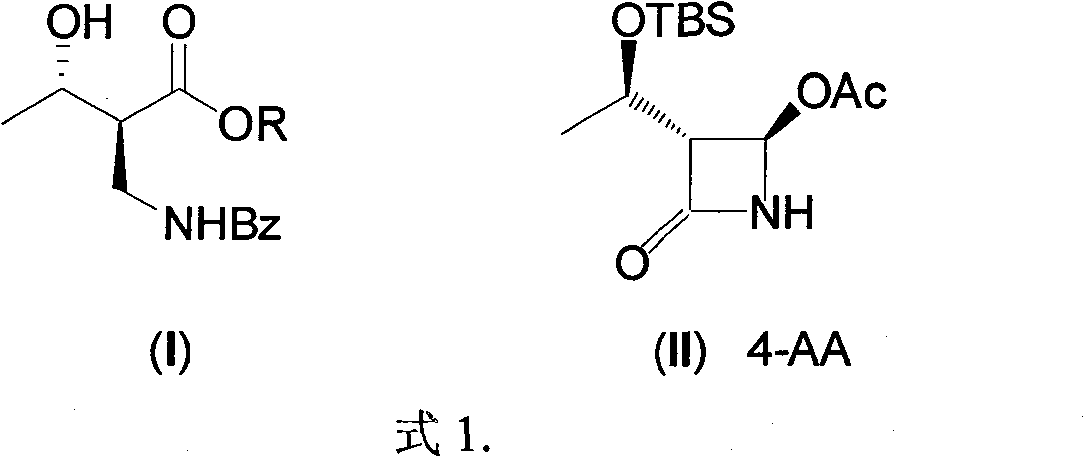
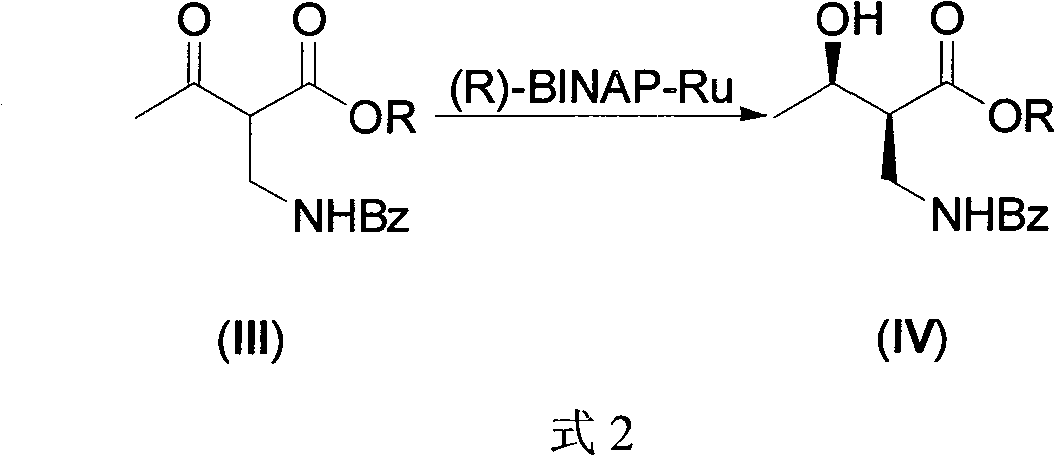
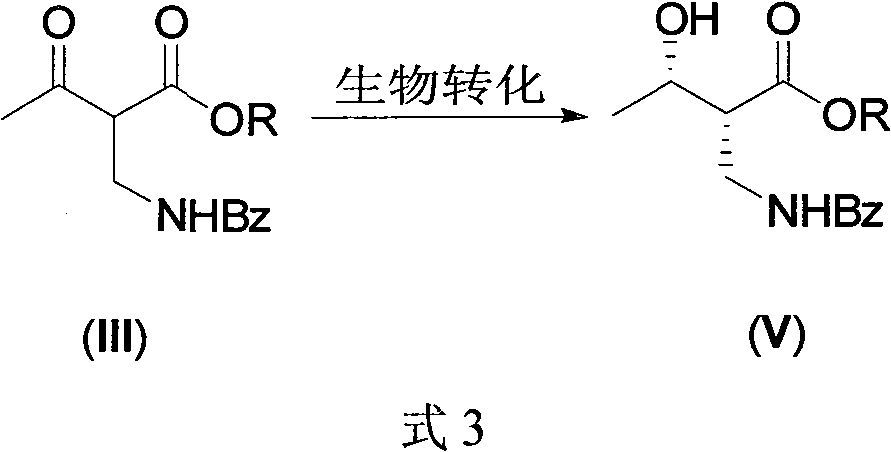
Synthesis of (2S,3S)-2-benzoyl aminometh-3-hydroxy-butyrate ester series compound by asymmetric yeast cell
A technology of benzoylaminomethyl and hydroxybutyrate, which is applied in the field of biological asymmetric synthesis of 2-benzoylaminomethyl-3-hydroxybutyrate, can solve the problem of high product yield and by-products Waste, environmental pollution and other problems, to achieve the effect of easy realization of reaction conditions, great competitive advantage, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] The preparation method of the present invention is further described in detail below by way of examples.
[0018] 1. (2S, 3S)-2-benzoylaminomethyl-3-hydroxybutyric acid ethyl ester is the compound of formula (I), (2R, 3S)-2-benzoylaminomethyl-3-hydroxybutyrate Ethyl acid ester is the preparation of formula (V) compound (R=Et):
[0019] Add tap water (2500ml), sucrose (600g), baker's yeast (100g, Angel Yeast, purchased in a supermarket) successively in a 5-liter three-necked round-bottomed flask equipped with a bubbler and a thermometer, and stir the mixture slightly (120r / min) .
[0020] After 1 hr, make the generated CO 2 Gas escapes at a rate of about 1 to 2 bubbles / second. Then 2-benzamidomethyl-3-carbonylbutyrate (III, 35 g) was added thereto, and the mixture was stirred at 33˜36° C. for 24 hours.
[0021] Sucrose (150 g) was dissolved in 500 ml of tap water and added to the reaction mixture, stirring was continued at 33-36° C., and the reaction was monitored by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com