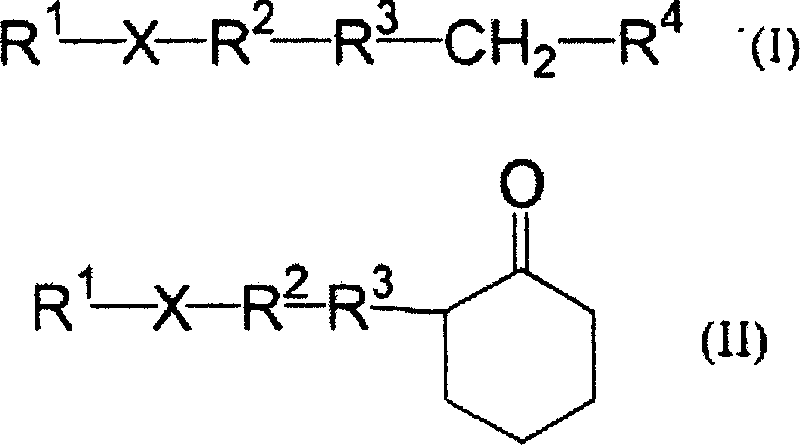
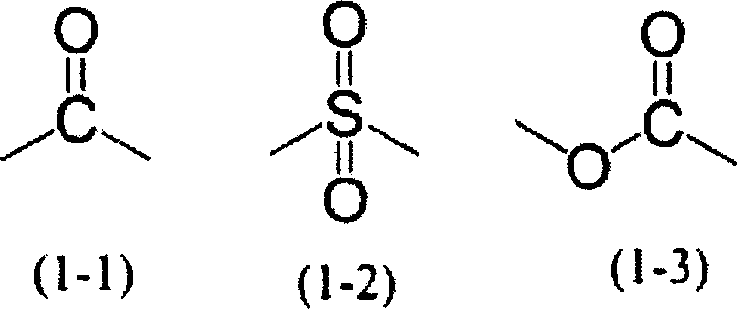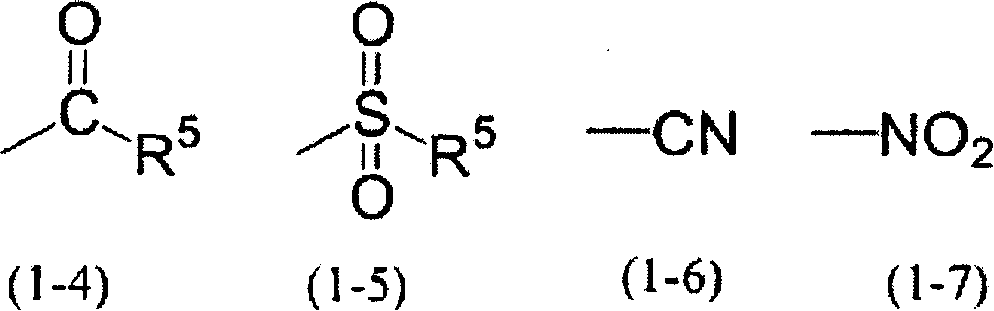Photosensitive resin composition
A technology of photosensitive resin and composition, applied in the field of photosensitive resin composition, can solve the problems of poor solvent resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0147] The present invention is explained in more detail below based on examples, but it is not meant that the present invention is limited by these examples. In the examples, unless otherwise specified, % and parts indicating content or usage-amount are based on weight.
Synthetic example 1
[0149]In a flask equipped with a reflux cooler, a dropping funnel, and a stirrer, 0.03 L / min was flowed with nitrogen as a nitrogen atmosphere, and 34.92 parts by weight of propylene glycol monomethyl ether acetate was added, and heated to 90° C. while stirring. Next, in 69.84 parts by weight of propylene glycol monomethyl ether acetate, 6.89 parts by weight of methacrylic acid, 34.28 parts by weight of 2-(methacryloyloxy) ethyl acetoacetate and 28.68 parts by weight of N -The polymerization initiator 2 of cyclohexylmaleimide, 3.28 parts by weight, 2'-azobis (isobutyronitrile), preparation solution, this solution is added dropwise to insulation at 90 ℃ by dropping funnel in 1 hour. in the flask at ℃. After the dropping of this solution was terminated, it was kept at 90° C. for 4 hours, and then cooled to room temperature to obtain a solution of a copolymer (resin Aa). The obtained resin Aa had a weight average molecular weight (Mw) of 14300 and a degree of dispersion of 2.45....
Synthetic example 2
[0151] In a flask equipped with a reflux cooler, a dropping funnel, and a stirrer, 0.03 L / min of nitrogen gas was flowed in as a nitrogen atmosphere, and 33.06 parts by weight of propylene glycol monomethyl ether acetate was added, and heated to 90° C. while stirring. Next, in 66.11 parts by weight of propylene glycol monomethyl ether acetate, 10.33 parts by weight of methacrylic acid, 34.28 parts by weight of 2-(methacryloyloxy) ethyl acetoacetate and 21.51 parts by weight of N -cyclohexylmaleimide, 3.28 parts by weight of the polymerization initiator 2,2'-azobis (isobutyronitrile) preparation solution, the solution was added dropwise to the solution at 90°C with a dropping funnel in 1 hour in the lower flask. After the dropwise addition of this solution was terminated, it was kept at 90° C. for 4 hours, and then cooled to room temperature to obtain a solution of a copolymer (resin Ab). The obtained resin Ab had a weight average molecular weight of 11900 and a degree of disp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com