20-hydroxy-16 alpha- bromo-steroid compound, synthesis method and its uses
A technology of steroidal compounds and synthetic methods, which is applied in the field of 20-hydroxy-16α-bromo-steroidal compounds, can solve the problems of complex products and low yield of androst-16-en-3β-ol, and achieve product High yield, low reagent price, avoid serious pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
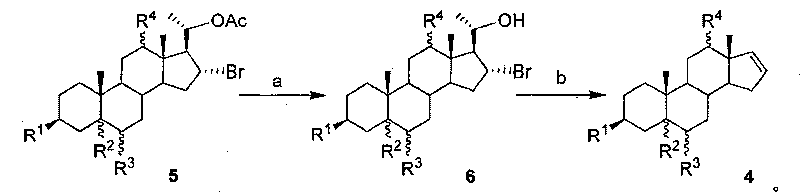
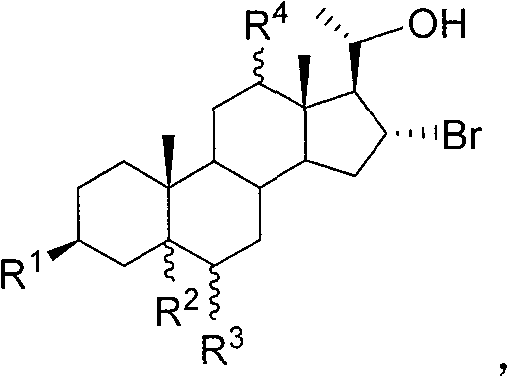
[0033] The synthesis of embodiment 1 compound 6a
[0034]
[0035] Weigh 500mg of compound 5a and dissolve it in an appropriate amount of MeOH, add 429mg (3eq.) K 2 CO 3 The hydrolysis reaction was carried out at 43° C. for 10 hours. Concentrate to near dryness, collect the residue with ethyl acetate, wash the organic phase with saturated brine, dry over anhydrous magnesium sulfate, let it stand for 30 min, filter, spin evaporate ethyl acetate, and obtain 178 mg of compound 6a (89.2%) by column chromatography.
[0036] Compound 6a, mp 168°C, [α] 25 D +9.2 (c 0.765, CHCl 3 ); 1 H-NMR (CDCl 3 , 400MHz) δ: 0.63(s, 3H, 18-H), 0.85(s, 3H, 19-H), 3.33(m, 1H, 3-H) 3.74(m, 1H, 20-H), 4.42( m, 1H, 16-H); IR v: 3415, 2921, 2847, 1442, 1372, 1041cm -1 ;Elemental analysis Calculated value C 25 h 39 o 4 Br: C 63.15, H 8.83; found C 63.36, H 8.47.
Embodiment 2
[0037] The synthesis of embodiment 2 compound 6d
[0038]
[0039] Weighed 500 mg of compound 5d and dissolved it in 10 mL of EtOH / THF (1:1), added 22 mg (1.0 eq.) of LiOH for hydrolysis reaction, and refluxed until the raw materials disappeared. After spinning to dryness, 345 mg of compound 6d (89.9%) was obtained by column chromatography.
[0040] Compound 6d, 1 H-NMR (CD 3 OD, 300MHz) δ: 0.86(s, 3H, 19-Me), 1.02(s, 3H, 18-Me), 1.41(d, J=6.8Hz, 3H, 21-Me), 3.24(dd, J= 11.1, 4.7Hz, 1H, 12-H), 3.50(m, 1H, 3-H), 4.38(m, 1H, 20-H), 4.57(m, 1H, 16-H); IR v: 3421, 1442, 1372, 1041cm -1 ;Elemental analysis Calculated value C 21 h 35 BrO 3 : C 60.72, H 8.49; Found C 59.96, H 8.68.
Embodiment 3
[0041] The synthesis of embodiment 3 compound 6e
[0042]
[0043] Weigh 500 mg of compound 5e and dissolve it in 8 mL of MeOH, add 255 mg (1.0 eq.) of Cs 2 CO 3 Carry out hydrolysis reaction, and react at room temperature until the raw materials disappear. Concentrate to near dryness, collect the residue with ethyl acetate, wash the organic phase with saturated brine, dry over anhydrous magnesium sulfate, let it stand for 30 min, filter, spin evaporate ethyl acetate, and obtain 298 mg of compound 6e (68.6%) by column chromatography.
[0044] Compound 6e, 1 H-NMR (CDCl 3 , 300MHz) δ: 0.75(s, 3H), 0.82(s, 3H), 1.35(d, J=5.2Hz, 3H), 3.53(m, 1H), 3.84(m, 1H), 4.54(m, 1H ), 4.81(m, 1H); elemental analysis calculated value C 21 h 33 Br 3 o 2 : C 45.27, H 5.97; Found C 45.02, H 5.63.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com