Somatostatin prodrugs
a technology of somatostatin and prodrug, which is applied in the direction of pharmaceutical delivery mechanism, peptide/protein ingredient, peptide/protein ingredient, etc., can solve the problems of poor intestinal permeability of somatostatin, require parenteral administration, etc., and achieve the effect of increasing the bioavailability of somatostatin compound, increasing the hydrophobicity and permeability, and without losing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on of Octreotide Prodrug
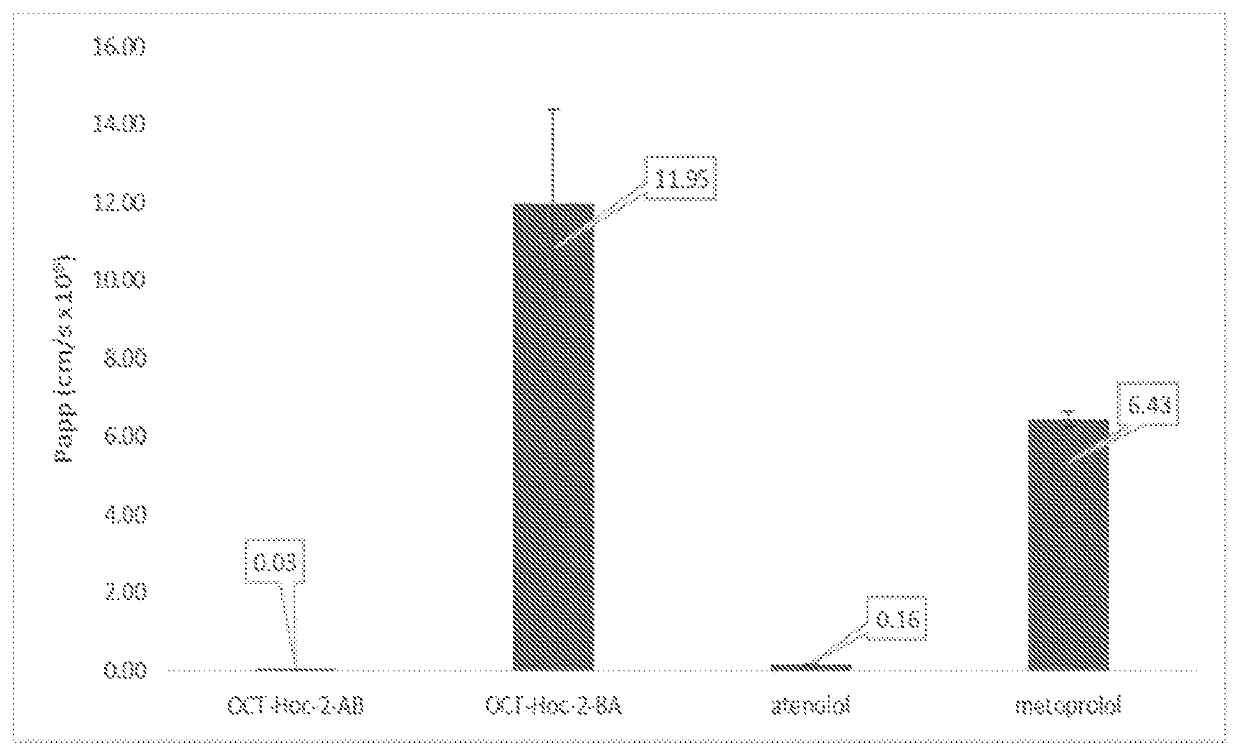
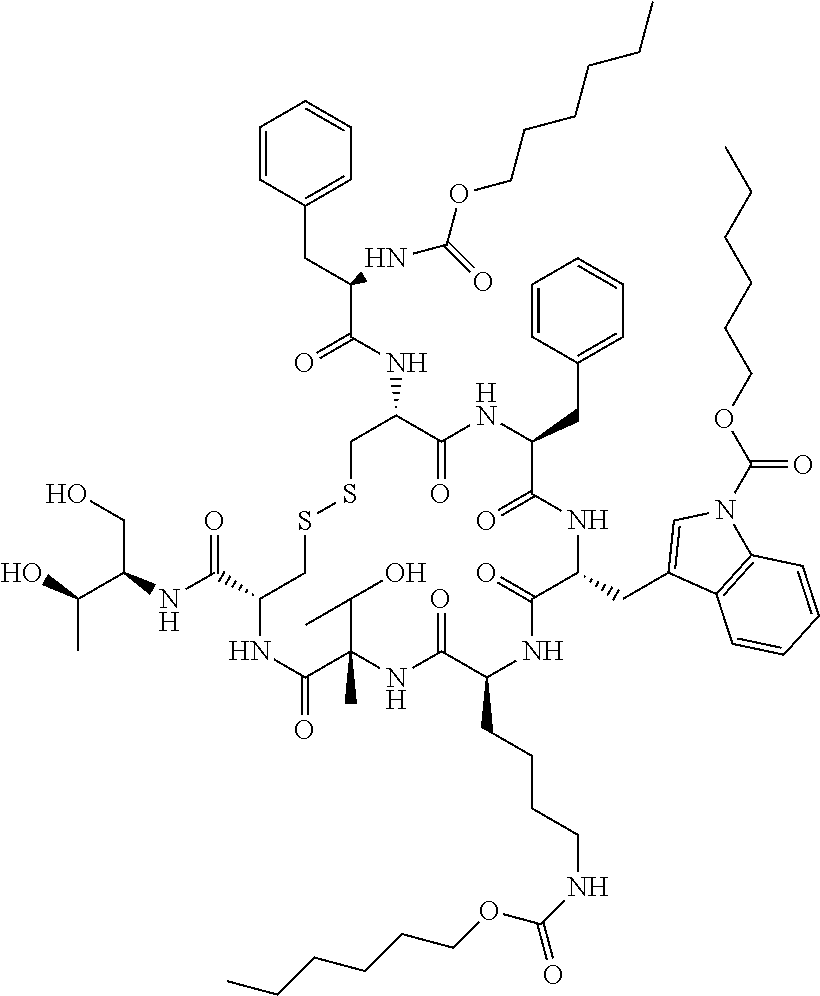
[0224]The prodrug three hexyloxycarbonyl-octreotide (Octreotide-P) was synthesized from octreotide using the synthetic pathway shown in Scheme 1:
[0225]
example 2
of Somato8 (Peptide 8) and its Prodrug Somato8-P (Peptide 8-P)
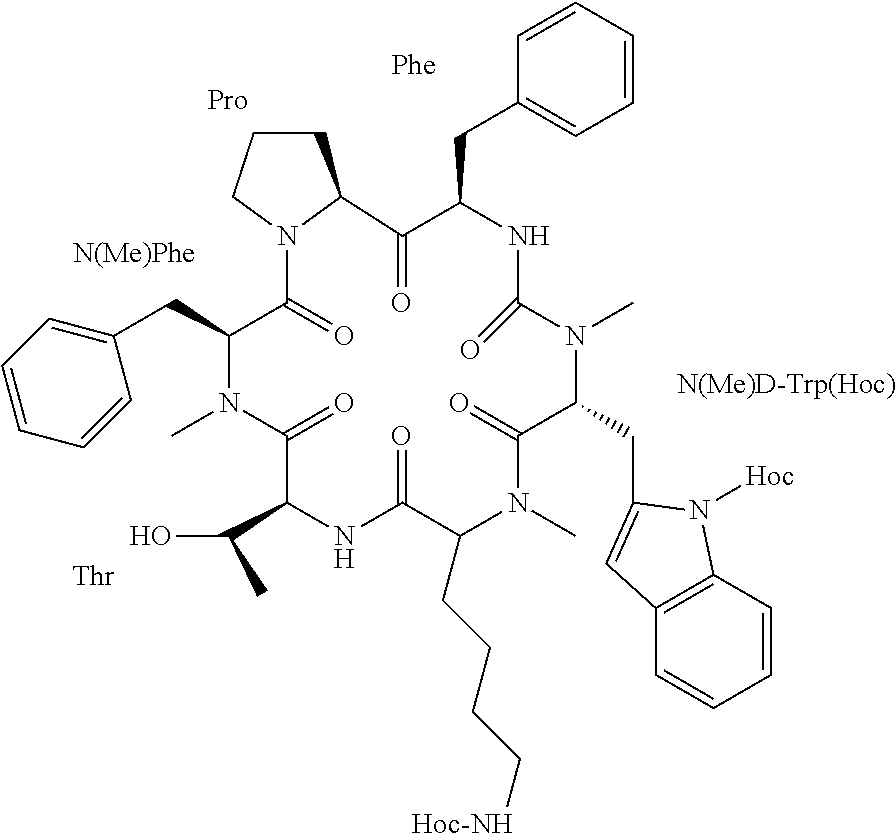
[0226]In an effort to develop an improved somatostatin analog, a cyclic N-methylated hexapeptide somatostatin analog denoted “Peptide 8” was selected from a combinatorial library of all possible N-methylated analogs of the potent hexa-cyclic somatostatin analog c(PFwKTF) (SEQ ID NO: 7) [31]. Out of the 30 analogs synthesized, only seven analogs were found to have somatostatin receptor (SSTR) affinity similar to that of the parent peptide, that is, selectivity towards SSTR2 and SSTRS in the nanomolar range. From these seven analogs, one, named “Somato8” (previously “Peptide 8”), having the sequence c(PF(NMe)w(NMe)KT(NMe)F) (SEQ ID NO: 8), that contains three N-methylated amino acid residues, had the most promising PK parameters in vitro (including stability to intestinal enzymes and intestinal permeability). It was further investigated for its bioavailability following oral administration to rats compared to the parent seq...
example 3
Cyclic Somatostatin Analogs and their Prodrugs
[0238]In an attempt to identify novel somatostatin analogs, libraries of backbone cyclic peptides have been previously prepared with compounds having identical or highly similar sequences to the somatostatin pharmacophoric sequences. Four libraries, each containing 96 compounds, were synthesized and screened for their binding affinities to somatostatin receptors. Following the screening process, several candidates were further investigated for their metabolic stability and pharmacodynamic profile compared to SRIF and to octreotide. Some of the compounds are PTR-3046 (SEQ ID NO: 9) [28], PTR-3205 (SEQ ID NO: 10) [29] and PTR-3173 (SEQ ID NO: 3) [30] depicted in Scheme 3:
[0239]
[0240]All backbone cyclic analogs were found to be stable against enzymatic degradation in serum and renal homogenate. However, their biological activity and selectivity varied toward the somatostatin receptors: while PTR-3046 was found to be selective toward the SST...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flow rate | aaaaa | aaaaa |
| surface area | aaaaa | aaaaa |
| apical volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com