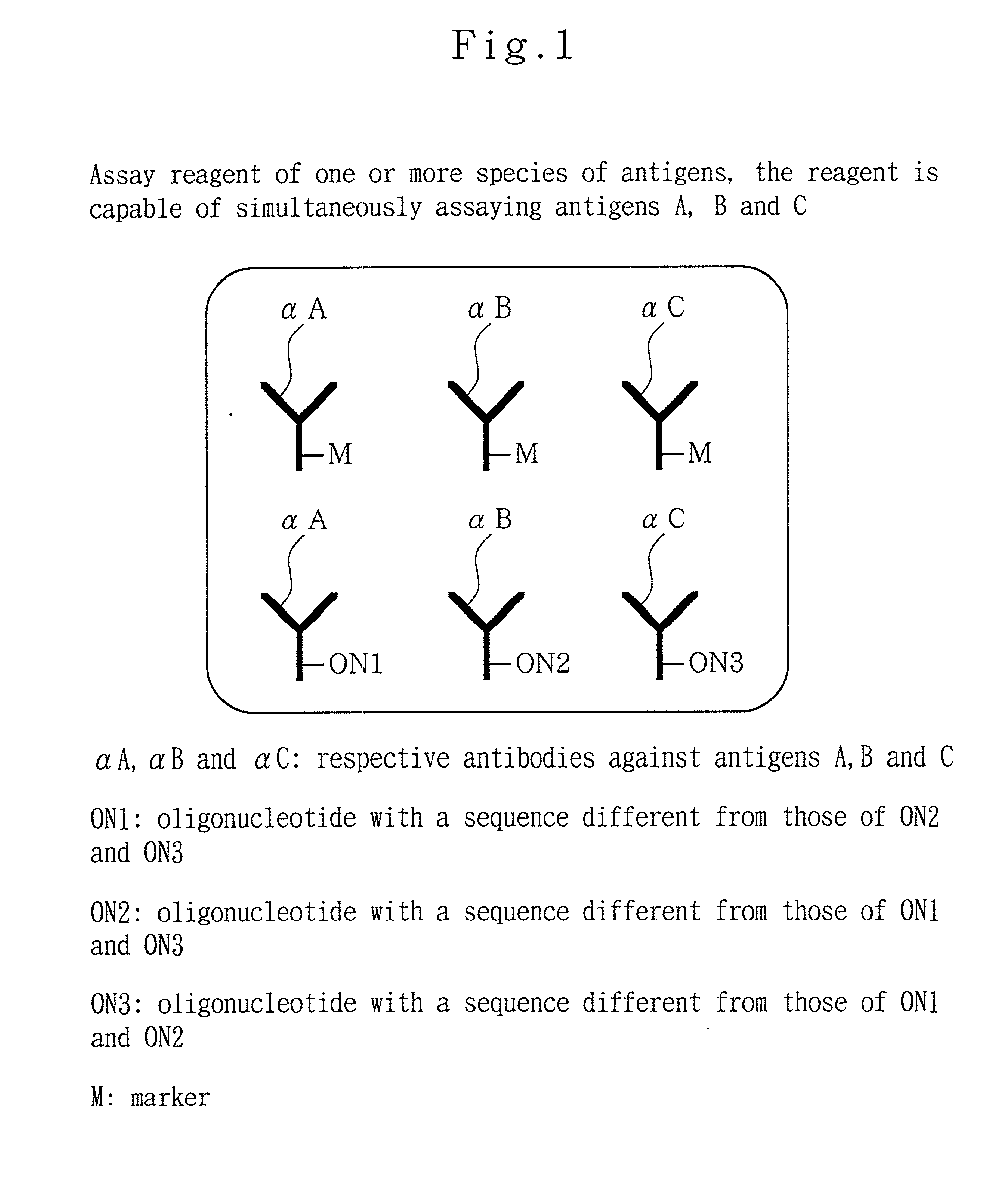
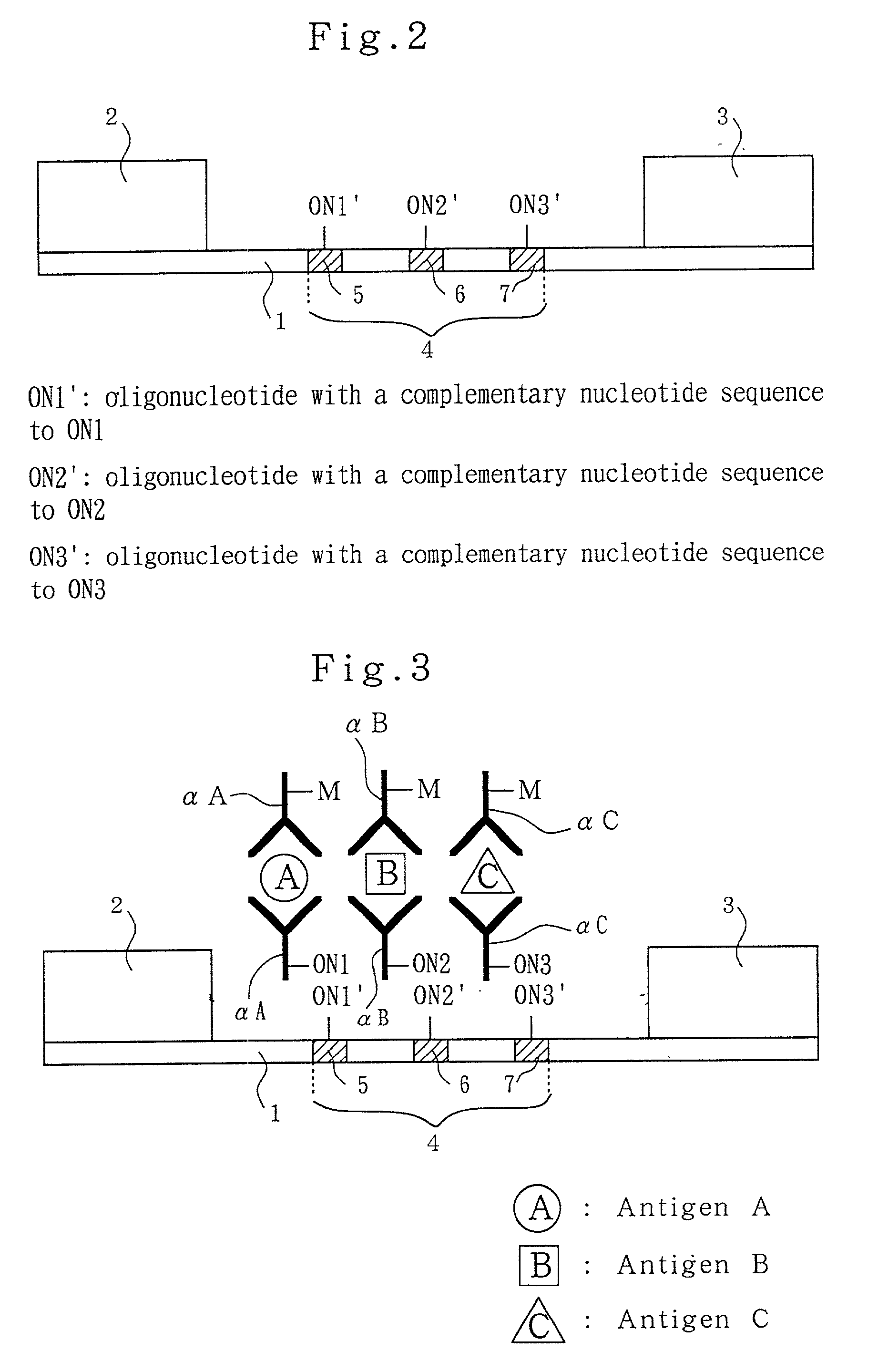
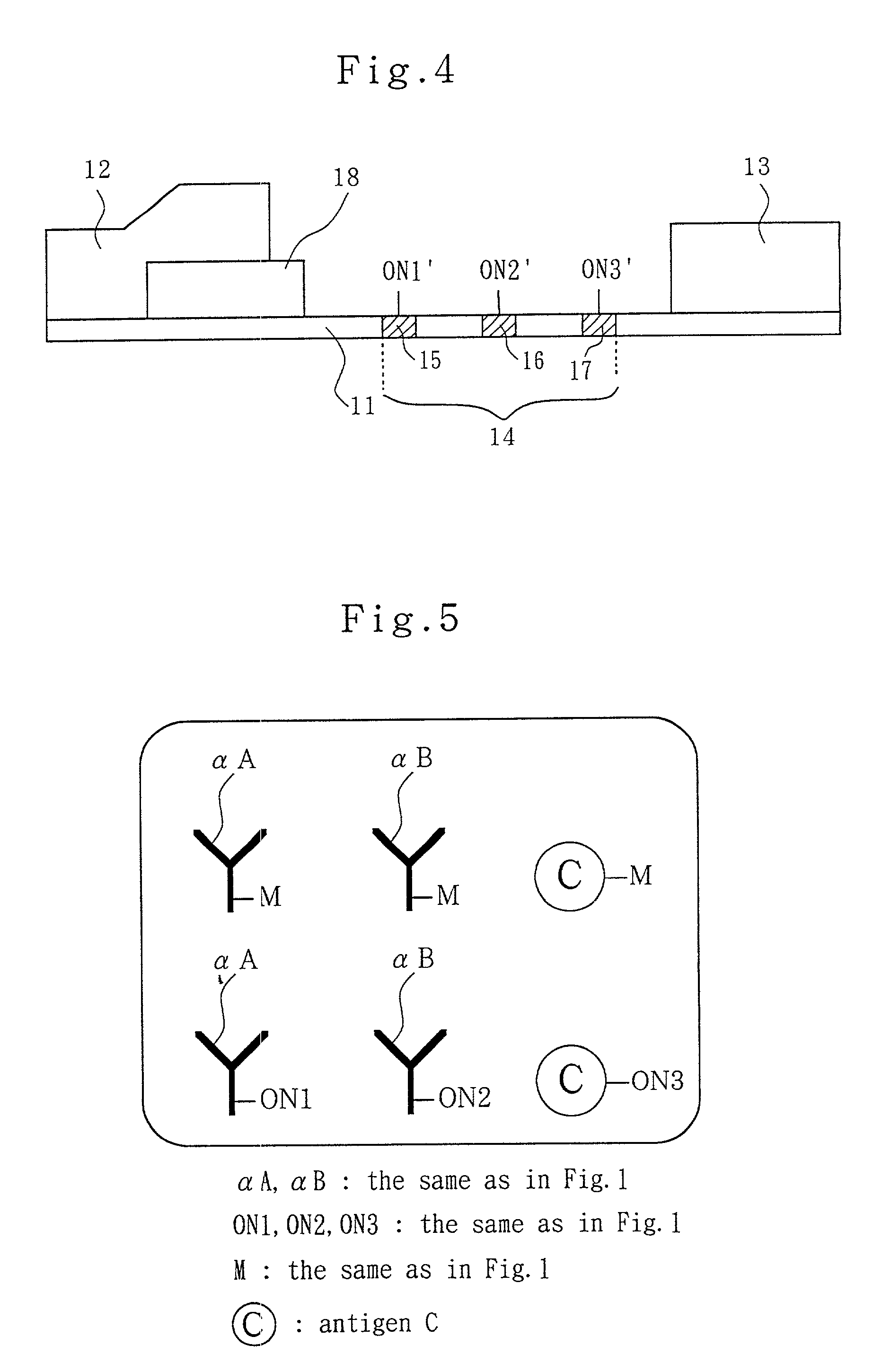
Analytical method, kit, and apparatus
a technology of kit and kit, applied in the field of assay method, can solve the problems of loss of treatment timing and lack of inclusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0085] Process 1: Preparation of biotinylated oligonucleotide
[0086] As shown below, oligonucleotides each having amino acid at 5' terminus were individually generated synthetically by using a DNA synthesizer, 391 A, manufactured by Perkin Elmer, Co.
[0087] Amino group--GAA TTC CCG GGG ATC CGT CG
[0088] (referred to as pair 1+ hereinafter)
[0089] Amino group--CGA CGG ATC CCC GGG AAT TTC
[0090] (referred to as pair 1- hereinafter)
[0091] Amino group--AAC GGA ATC TAA TCA GGA GG
[0092] (referred to as pair 8+ hereinafter)
[0093] Amino group--CCT CCT GAT TAG ATT CCG TT
[0094] (referred to as pair 8- hereinafter).
[0095] These oligonucleotides each of 300 nmol were dissolved in 0.1 M MOPS buffer, pH 7.0 containing 1 mM EDTA, to which was added N-hydroxysuccinimide-biotin (30 .mu.mol; NHS-Biotin manufactured by Pierce, Co.) dissolved in N',N'-dimethylformamide (referred to as DMF hereinbelow), for reaction at 37.degree.C. for one hour. After the reaction, biotinylated oligonucleotides were separate...
example 2
[0115] Process 1: Preparation of (pair 1+)-labeled avidin and (pair 1-)-labeled avidin
[0116] The biotin-labeled pair 1+ (112 nmol) prepared at the process 1 in Example 1 was reacted with avidin (1.52 mg) in MPBS (0.7 ml) at 37.degree.C. for 3 hours, and subsequently, the mixture was loaded to Ultrogel AcA44 resin manufactured by IBF biotechniqes, preliminarily equilibrated with PBS, to recover pair 1+ labeled avidin. By the same method, additionally, pair 1+ labeled avidin was recovered.
[0117] Process 2: Preparation of oligonucleotide-avidin matrix-bound membrane
[0118] Avidin (10 mg) was dissolved in phosphate buffered physiological saline (manufactured by Nissui Pharmaceuticals, Co. PBS (-)),and then, membrane cut into a piece of 5.times.10 cm (SPHF membrane, manufactured by Millipore, Co.) was immersed in the solution at ambient temperature for one hour and was then rinsed with distilled water. After rinsing, the membrane piece was dried in air. By using a soft pen (manufactured b...
example 3
[0121] Process 1: Preparation of (pair 1-)-bound SPHF membrane
[0122] By using a soft pen (manufactured by Platinum Fountain Pen Co.) impregnated with 2 mg / ml (pair 1-)-labeled BSA as prepared at the process 4 in Example 1, a line was drawn vertically to the 5-cm side of a membrane cut into a size of 5.times.10 cm (SPHF membrane; manufactured by Millipore, Co.), to divide the side in halves, to bind the BSA through physical adsorption to the membrane. After drying in air, blocking by means of a blocking agent (Block Ace; manufactured by Snow Brand Milk Products, Co.) was effected at ambient temperature for 30 minutes, and subsequently, the resulting membrane was rinsed with distilled water and dried in air, to prepare (pair 1-)-bound SPHF membrane. After drying the membrane in air, the membrane was cut into a piece of a 0.5-cm width and a 5-cm length, and at one end of the piece was fixed GF with a staple, and storage under dry conditions.
[0123] Process 2: Capture of sandwich immunoc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com